Abstract
The quest for effective treatment for osteoporosis merits great attention because of the widespread prevalence of this disease, which is not only associated with fragility fractures, but also with significant morbidity and mortality. The efficacy of the antiresorptive drugs in this disease is achieved by reducing bone turnover, increasing bone density and improving other aspects of bone quality. This article concentrates on another approach to the treatment of osteoporosis, namely the use of anabolic therapy, which has even greater prospects for improving bone quality.
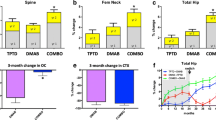
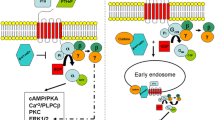
Parathyroid hormone (PTH) is currently available only as the recombinant amino-terminal fragment, PTH(1–34), known as teriparatide. The full-length molecule, human PTH(1–84), is currently being investigated, as are other PTH molecules. Teriparatide improves bone quality through actions on bone turnover, bone density, bone size and bone microarchitecture. In postmenopausal women with osteoporosis, teriparatide reduces the incidence of vertebral and nonvertebral fractures. In individuals who have previously been treated with an antiresorptive agent, the subsequent actions of teriparatide on bone density are transiently delayed if bone turnover has been markedly suppressed. Combination therapy with teriparatide or PTH(1–84) and an antiresorptive agent does not appear, at this time, to offer advantages over the use of PTH or an antiresorptive agent alone. However, in order to maintain the densitometric gains in bone density obtained with PTH, it is important to follow its use with that of an antiresorptive agent.






Similar content being viewed by others
References
Lindsay R, Cosman F. The pharmacology of estrogens in osteoporosis. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego (CA): Academic Press, 1996: 1063–8
Fleisch H. Bisphosphonates: mechanisms of action and clinical use. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego (CA): Academic Press, 1996: 1037–52
Azria M, Avioli L. Calcitonin. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego (CA): Academic Press, 1996: 1083–98
Fogelman I, Ribot C, Smith R, et al. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 2000; 85(5): 1895–900
Greenspan SL, Parker RA, Ferguson L, et al. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 1998; 13(9): 1431–8
Chesnut IC, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004; 19(8): 1241–9
Delmas PD, Recker RR, Chesnut CH, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 2004; 15(10): 792–8
Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000; 21(2): 115–37
Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 1999; 104(10): 1363–74
Seeman E. Bone quality. Osteoporos Int 2003; 14 Suppl. 5: 3–7
McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 2005; 165(15): 1762–8
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344(19): 1434–41
Cosman F, Shen V, Herrington B, et al. Response of the parathyroid gland to infusion of human parathyroid hormone-(1–34) [PTH-(1–34)]: demonstration of suppression of endogenous secretion using immunoradiometric intact PTH-(1–84) assay. J Clin Endocrinol Metab 1991; 73(6): 1345–51
Teriparatide injection (rDNA origin). Data on file, Eli Lilly, USA, 2001 [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/01/slides/3761s2_01_lilly [Accessed 2005 Aug 17]
Schwietert HR, Groen EW, Sollie FA, et al. Single-dose subcutaneous administration of recombinant human parathyroid hormone [rhPTH (1–84)] in healthy postmenopausal volunteers. Clin Pharmacol Ther 1997; 61(3): 360–76
Fraher L. A comparison of the pharmacokinetics of PTH in healthy young and osteoporotic subjects. J Bone Miner Res 1993; 8S: 253
Henry JG, Mitnick M, Dann PR, et al. Parathyroid hormonerelated protein-(1–36) is biologically active when administered subcutaneously to humans. J Clin Endocrinol Metab 1997; 82(3): 900–6
Forteo 750mcg/3ml solution 3ml syringe (teriparatide) [online]. Available from URL: http://www.drugstore.com/pharmacy/prices/drugprice.asp#00002897101 [Accessed 2005 Sep 2]
Product monograph: Forteo. Canada: Eli Lilly, 2004
Guyatt GH, Cranney A, Griffith L, et al. Summary of metaanalyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am 2002; 31(3): 659–79, xii
Marcus R, Wang O, Satterwhite J, et al. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 2003; 18(1): 18–23
Delmas P, Licata A, Crans G, et al. Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. J Bone Miner Res 2004; 19 Suppl. 1: 1170
Mosekilde L, Sogaard CH, Danielsen CC, et al. The anabolic effects of human parathyroid hormone (hPTH) on rat vertebral body mass are also reflected in the quality of bone, assessed by biomechanical testing: a comparison study between hPTH-(1–34) and hPTH-(1–84). Endocrinology 1991; 129(1): 421–8
Kimmel DB, Bozzato RP, Kronis KA, et al. The effect of recombinant human (1–84) or synthetic human (1–34) parathyroid hormone on the skeleton of adult osteopenic ovariectomized rats. Endocrinology 1993; 132(4): 1577–84
Reeve J, Meunier PJ, Parsons JA, et al. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. BMJ 1980; 280(6228): 1340–4
Paschalis EP, Burr DB, Mendelsohn R, et al. Bone mineral and collagen quality in humeri of ovariectomized cynomolgus monkeys given rhPTH (1–34) for 18 months. J Bone Miner Res 2003; 18(4): 769–75
Mashiba T, Burr DB, Turner CH, et al. Effects of human parathyroid hormone (1–34), LY333334, on bone mass, remodeling, and mechanical properties of cortical bone during the first remodeling cycle in rabbits. Bone 2001; 28(5): 538–47
Dempster DW, Parisien M, Silverberg SJ, et al. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 1999; 84(5): 1562–6
Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 2001; 16(10): 1846–53
Turner CH, Burr DB, Hock JM, et al. The effects of PTH (1–34) on bone structure and strength in ovariectomized monkeys. Adv Exp Med Biol 2001; 496: 165–79
Zanchetta JR, Bogado CE, Ferretti JL, et al. Effects of teriparatide [recombinant human parathyroid hormone (1–34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 2003; 18(3): 539–43
Body JJ, Gaich GA, Scheele WH. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002; 87(10): 4528–35
Hodsman AB, Hanley DA, Ettinger MP, et al. Efficacy and safety of human parathyroid hormone-(1–84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 2003; 88(11): 5212–20
Recker R, Bare S, Miller M, et al. Treatment of osteoporotic women with parathyroid hormone 1–84 for 18 months improves cancellous bone formation and structure; a bone biopsy study. J Bone Miner Res 2004; 19 Suppl. 1: S97
Ettinger M, Greenspan S, Marriott TB, et al. PTH (1–84) prevents first vertebral fracture in postmenopausal women with osteoporosis: results from the TOP study [abstract]. American College of Rheumatology 68th Annual Scientific Meeting; 2004 Oct 16–21; San Antonio (TX), L17
Kurland ES, Cosman F, McMahon DJ, et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 2000; 85(9): 3069–76
Rubin M, Bilezikian J. The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med 2002; 19: 415–32
Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 2003; 18(1): 9–17
Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 2005 May; 16(5): 510–6
Wehrli FW, Hilaire L, Fernandez-Seara M, et al. Quantitative magnetic resonance imaging in the calcaneus and femur of women with varying degrees of osteopenia and vertebral deformity status. J Bone Miner Res 2002; 17(12): 2265–73
Genant HK, Li J, Wu CY, et al. Vertebral fractures in osteoporosis: a new method for clinical assessment. J Clin Densitom 2000; 3(3): 281–90
Parfitt AM, Mathews CH, Villanueva AR, et al. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis: implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest 1983; 72(4): 1396–409
Silva MJ, Gibson LJ. Modeling the mechanical behavior of vertebral trabecular bone: effects of age-related changes in microstructure. Bone 1997; 21(2): 191–9
Jiang Y, Zhao JJ, Mitlak BH, et al. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 2003; 18(11): 1932–41
Eriksen EF. Primary hyperparathyroidism: lessons from bone histomorphometry. J Bone Miner Res 2002; 17 Suppl. 2: N95–7
Aaron JE, de Vernejoul MC, Kanis JA. Bone hypertrophy and trabecular generation in Paget’s disease and in fluoride-treated osteoporosis. Bone Miner 1992; 17(3): 399–413
Jerome CP, Burr DB, Van Bibber T, et al. Treatment with human parathyroid hormone (1–34) for 18 months increases cancellous bone volume and improves trabecular architecture in ovariectomized cynomolgus monkeys (Macaca fascicularis). 2001; 28(2): 150–9
Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997; 350(9077): 550–5
Burr DB, Hirano T, Turner CH, et al. Intermittently administered human parathyroid hormone (1–34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humérus of ovariectomized cynomolgus monkeys. J Bone Miner Res 2001; 16(1): 157–65
Hodsman AB, Kisiel M, Adachi JD, et al. Histomorphometric evidence for increased bone turnover without change in cortical thickness or porosity after 2 years of cyclical hPTH (1–34) therapy in women with severe osteoporosis. Bone 2000; 27(2): 311–8
Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 2001; 16(5): 925–31
Roe E, Sanchez S, del Puerto G, et al. Parathyroid hormone 1–34 (hPTH 1–34) and estrogen produce dramatic bone density increases in postmenopausal osteoporosis: results from a placebo-controlled randomized trial [abstract]. J Bone Miner Res 1999; 14 Suppl. 1: S137
Ettinger B, San Martin J, Crans G, et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004; 19(5): 745–51
Cosman F, Nieves J, Woelfert L, et al. Alendronate does not block the anabolic effect of PTH in postmenopausal osteoporotic women. J Bone Miner Res 1998; 13(6): 1051–5
Cosman F, Nieves J, Zion M, et al. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 2005; 353(6): 566–75
Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003; 349(13): 1207–15
Finkelstein JS, Hayes A, Hunzelman JL, et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 2003; 349(13): 1216–26
Deal C, Omizo M, Schwartz E, et al. Raloxifene in combination with teriparatide reduced teriparatide-induced stimulation of bone resorption but nor formation in postmenopausal women with osteoporosis [abstract]. J Bone Miner Res 2004; 19 Suppl. 1: 1169
Onyia J. Gene array analysis of the bone effects of raloxifene and alendronate show that alendronate strongly inhibits the expression of bone formation marker genes [abstract]. J Bone Miner Res 2002; 17 Suppl. 1: S157
Katz R, Sun Q, Bilezikian J, et al. Bisphosphonates differentially affect osteoblast survival in vitro [abstract]. J Bone Miner Res 2004; 19 Suppl. 1: S477
Watts N, McClung M, Olsynski W, et al. Effects of risedronate discontinuation on bone turnover and bone mineral density in postmenopausal women with osteoporosis [abstract]. J Clin Densitom 2004; 7: 37
Delmas PD, Vergnaud P, Arlot ME, et al. The anabolic effect of human PTH (1–34) on bone formation is blunted when bone resorption is inhibited by the bisphosphonate tiludronate: is activated resorption a prerequisite for the in vivo effect of PTH on formation in a remodeling system? Bone 1995; 16(6): 603–10
Rubin MR, Bilezikian JP. Clinical review 151. The role of parathyroid hormone in the pathogenesis of glucocorticoidinduced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab 2002; 87(9): 4033–41
Lane NE, Sanchez S, Modin GW, et al. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis: results of a randomized controlled clinical trial. J Clin Invest 1998; 102(8): 1627–33
Buxton EC, Yao W, Lane NE. Changes in serum receptor activator of nuclear factor-kappaB ligand, osteoprotegerin, and interleukin-6 levels in patients with glucocorticoid-induced osteoporosis treated with human parathyroid hormone (1–34). J Clin Endocrinol Metab 2004; 89(7): 3332–6
Neer R, Arnaud CD, Zanchetta J, et al. Recombinant human PTH [rhPTH (1–34)] reduces the risk of spine and non-spine fractures in postmenopausal osteoporosis [abstract]. 82nd Annual Meeting of the Endocrine Society; 2000 Jun 21–24; Toronto (ON)
Rittmaster RS, Bolognese M, Ettinger MP, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 2000; 85(6): 2129–34
Lindsay R, Scheele WH, Clancy AD, et al. Reduction in nonvertebral fragility fractures and increase in spinal bone density is maintained 31 months after discontinuation of recombinant human parathyroid hormone (1–34) in postmenopausal women with osteoporosis [abstract no. OR35-6]. 84th Annual Meeting of the Endocrine Society; 2002 Jun 19–22; San Francisco (CA), 113
Lane NE, Sanchez S, Modin GW, et al. Bone mass continues to increase at the hip after parathyroid hormone treatment is discontinued in glucocorticoid-induced osteoporosis: results of a randomized controlled clinical trial. J Bone Miner Res 2000; 15(5): 944–51
Kurland ES, Heller SL, Diamond B, et al. The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone (1–34)]. Osteoporos Int 2004; 15(12): 992–7
Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 2005; 353(6): 555–65
Misof BM, Roschger P, Cosman F, et al. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab 2003; 88(3): 1150–6
FORTEO (teriparatide [rDNA origin] injection) [package insert]. Indianapolis (IN): Eli-Lilly, 2002: 1
Vahle JL, Long GG, Sandusky G, et al. Bone neoplasms in F344 Rats given teriparatide [rhPTH (1–34)] are dependent on duration of treatment and dose. Toxicol Pathol 2004; 32(4): 426–38
Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol 2002; 30(3): 312–21
Wilker C, Jolette J, Smith S, et al. A no observable carcinogenic effect dose level identified in Fischer 344 rats following daily treatment with PTH (1–84) for 2 years: role of the C-terminal PTH receptor? [abstract]. J Bone Miner Res 2004; 19 Suppl. 1: SA435
Tashjian Jr AH, Chabner BA. Commentary on clinical safety of recombinant human parathyroid hormone 1–34 in the treatment of osteoporosis in men and postmenopausal women. J Bone Miner Res 2002; 17(7): 1151–61
Betancourt M, Wirfel KL, Raymond AK, et al. Osteosarcoma of bone in a patient with primary hyperparathyroidism: a case report. J Bone Miner Res 2003; 18(1): 163–6
Wiig JN, Bakken TS. Hyperparathyroidism with multiple malignant tumours of bone with giant-cells: a case report. Acta Chir Scand 1971; 137(4): 391–3
Smith J, Huvos AG, Chapman M, et al. Hyperparathyroidism associated with sarcoma of bone. Skeletal Radiol 1997; 26(2): 107–12
Palmer M, Adami HO, Krusemo UB, et al. Increased risk of malignant diseases after surgery for primary hyperparathyroidism: a nationwide cohort study. Am J Epidemiol 1988; 127(5): 1031–40
Gopalakrishnan V, Hwang S, Loughre H, et al. Administration of ThPTH to humans using Macroflux transdermal technology reults in the rapid delivery of biologically active PTH [abstract]. J Bone Miner Res 2004; 19 Suppl. 1: M484
Leone-Bay A, Sato M, Paton D, et al. Oral delivery of biologically active parathyroid hormone. Pharm Res 2001; 18(7): 964–70
Mehta NM, Gilligan J, Stern B, et al. Biological activity of recombinant PTH analog 7841. J Bone Miner Metab 2002; 17 Suppl. 1: SA362
Fraher LJ, Avram R, Watson PH, et al. Comparison of the biochemical responses to human parathyroid hormone-(l–31) NH2 and hPTH-(l–34) in healthy humans. J Clin Endocrinol Metab 1999; 84(8): 2739–43
Horwitz MJ, Tedesco MB, Gundberg C, et al. Short-term, highdose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 2003; 88(2): 569–75
Black DM, Rosen CJ. Parsimony with PTH: is a single weekly injection of PTH superior to a larger cumulative dose given daily? [abstract]. J Bone Miner Res 2002; 17 Suppl. 1: SA367
Gowen M, Stroup GB, Dodds RA, et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J Clin Invest 2000; 105(11): 1595–604
Acknowledgements
This project was funded by NIH grant NIDDK 32333.
Drs Rubin and Bilezikian have no conflicts of interest that are directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rubin, M.R., Bilezikian, J.P. Parathyroid Hormone as an Anabolic Skeletal Therapy. Drugs 65, 2481–2498 (2005). https://doi.org/10.2165/00003495-200565170-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565170-00005