Abstract
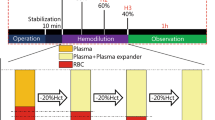
Objective: Using an isovolaemic model, the effect on the blood and plasma volumes of a newly developed medium-molecular weight (130 Kd) hydroxyethyl starch (HES) solution (degree of substitution 0.4) was investigated in 12 healthy male volunteers with the aid of 51Cr-radiolabelled erythrocytes.
Methods: Blood samples were drawn before and after 500ml bleeding as well as directly prior to the start of a 500ml HES infusion, and repeatedly up to 24 hours after treatment, for the measurement of haematocrit and gamma-counting, and the calculation of blood and plasma volumes.
Results: The blood and plasma volumes increased by a maximum of 0.385L (7%) and 0.620L (21%), respectively, at 30 minutes after the start of infusion, and returned to the baseline value at 24 hours after infusion. The plasma volume exceeded the volume prior to the start of bleeding by more than 5% for 19.15 hours. A clinically relevant expansion of the plasma volume persisted for at least 6 hours after the infusion and was comparable to the infused volume. The red blood cell volume remained stable after bleeding [mean: 2.01L; SD: 0.03L (range 1.95L to 2.05L)]. Local tolerability at the infusion site and systemic tolerability were good. Blood pressure, heart rate and ECG did not show clinically relevant deviations from normal.
Conclusion: It is concluded that a blood withdrawal of 500ml can be replaced isovolaemically by 500ml of the new 6% HES (130/0.4) solution.
Similar content being viewed by others
References
Ferber HP, Nitsch E, Förster H. Studies on hydroxyethyl starch. Pt II. Changes of the molecular weight distribution for hydroxyethyl starch types 450/0.7,450/0.5,450/0.3, 300/0.4, 200/0.7, 200/0.5, 200/0.3 and 200/0.1 after infusion in serum and urine of volunteers. Arzneimittel Forschung 1985; 35: 615–22
Weidler B, von Bormann B, Sommermeyer K, et al. Pharmakokinetische Merkmale als Kriterien für den klinischen Einsatz von Hydroxyethylstärke. Arzneimittel Forschung 1991; 41(5): 494–8
Jung F, Koscielny J, Mrowietz C, et al. Einfluβ der Molekülstruktur von Hydroxyäthylstärke auf die Eliminationskinetik und die Flieβfähigkeit des Blutes bei Probanden. Arzneimittel Forschung 1993;, 43: 99–105
Jung F, Koscielny J, Mrowietz C, et al. Elimination kinetics of different hydroxyethyl starches and effects on blood fluidity. Clin Hemorheol 1994; 14: 189–202
Köhler H, Zschiedrich H, Linfante A, et al. Die Elimination von Hydroxyäthylstärke 200/0.5, Dextran 40 und Oxypolygelatine. Klin Wochenschr 1982; 60: 293–301
Moertelmans Y, Merckx E, van Nerom C, et al. Effect of an equal volume replacement with 500ml 6% hydroxyethyl starch on the blood and plasma volume of healthy volunteers. Eur J Anaesthesiol 1995; 12: 259–64
Treib J, Haass A, Pindur G, et al. Blutungskomplikationen durch Hydroxyethylstärke sind vermeidbar. Dt Ärztebl 1997; 94: A–2326–30
Waitzinger J, Bepperling F, Pabst G, et al. Pharmacokinetics and tolerability of a new hydroxyethyl starch (HES) specification [HES (130/0.4)] after single-dose infusion of 6% or 10% solutions in healthy volunteers. Clin Drug Invest 1998; 16(2): 151–60
International Committee for Standardisation in Haematology (ICSH). Panel on diagnostic applications of radioisotopes in haematology. Br J Haematol 1973; 25: 801–14
International Committee for Standardisation in Haematology. Recommended Method for Radioisotope Red-Cell Survival Studies. Br J Haematol 1980; 45: 659–66
International Committee for Standardisation in Haematology. Recommended Methods for Measurement of Red-Cell and Plasma Volume. J Nucl Med 1980; 21: 793–800
Voak D, Finney RD, Forman K, et al. Guidelines for autologous transfusion. I. Pre-operative autologous donation. Trans Med 1993; 3: 307–16
Dahlmann H, Kasper M. Volumenersatz bei der Eigenblutspende — Pro und Kontra. Beitr Infusionsther Basel, Karger 1993; 29: 190–6
Lundvall J, Bjerkhoel P. Failure of hemoconcentration during Standing to reveal plasma volume decline induced in the erect posture. J Appl Physiol 1994; 77: 2155–62
Kröll W, Gerner P, Colombo T, et al. Einfluβ von 6% HES 200/0.6–0.66 auf Plasmavolumen und Blutgerinnung. Infusionsther-Transfusionsmed 1992; 19: 171–80
Halmagyi M. Zur Bewertung des kolloidalen Volumenersatzmittels 6% HAS 40/0.5. Anästhesist 1984; 33: 73–81
Degrémont AC, Ismail M, Arthaud M, et al. Mechanism of postoperative prolonged plasma volume expansion with low molecular weight hydroxyethyl starch (HES 200/0.62, 6%). Intensive Care Med 1995; 21: 577–83
Spahn DR, Leone BJ, Reves JG, et al. Cardivascular and coronary physiology of acute isovolemic hemodilution: a review of nonoxygen-carrying and oxygen-carrying solutions. Anesth Analg 1994; 78: 1000–21
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waitzinger, J., Bepperling, F., Pabst, G. et al. Effect of a New Hydroxyethyl Starch (HES) Specification [6% HES (130/0.4)] on Blood and Plasma Volumes after Bleeding in 12 Healthy Male Volunteers. Clin. Drug Investig. 17, 119–125 (1999). https://doi.org/10.2165/00044011-199917020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199917020-00006