Abstract
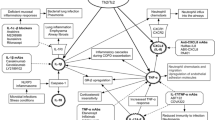
Chronic obstructive pulmonary disease (COPD) and asthma are inflammatory diseases of the lung where a hallmark feature is excessive leukocyte infiltration that leads to tissue injury. Cell adhesion molecules (e.g. selectins and integrins) play a key role in cell trafficking, and in the lung they regulate leukocyte extravasation, migration within the interstitium, cellular activation, and tissue retention. All selectin family members (including L-selectin, P-selectin, and E-selectin) and many of the β1 and β2 integrins appear to be important therapeutic targets, as numerous animal studies have demonstrated essential roles for these cell adhesion molecules in lung inflammation. Not surprisingly, these families of adhesion molecules have been under intense investigation by the pharmaceutical industry for the development of novel therapeutics. Integrins are validated drug targets, as drugs that antagonize integrin αIIbβ3 (e.g. abciximab), integrin αLβ2 (efalizumab), and integrin α4β1 (natalizumab) are currently US FDA-approved for acute coronary syndromes, psoriasis, and multiple sclerosis, respectively. However, none has been approved for indications related to asthma or COPD. Here, we provide an overview of roles played by selectins and integrins in lung inflammation. We also describe recent clinical results (both failures and successes) in developing adhesion molecule antagonists, with specific emphasis on those targets that may have potential benefit in asthma and COPD. Early clinical trials using selectin and integrin antagonists have met with limited success. However, recent positive phase II clinical trials with a small-molecule selectin antagonist (bimosiamose) and a small-molecule integrin α4β1 antagonist (valategrast [R411]), have generated enthusiastic anticipation that novel strategies to treat asthma and COPD may be forthcoming.




Similar content being viewed by others
References
Tantisira KG, Weiss ST. The pharmacogenetics of asthma therapy. Curr Drug Targets 2006; 7: 1697–708
Decramer M, Ferguson G. Clinical safety of long-acting beta2-agonist and inhaled corticosteroid combination therapy in COPD. COPD 2006; 3: 163–71 © 2008 Adis Data Information BV. All rights reserved.
Mallia P, Contoli M, Caramori G, et al. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr Pharm Des 2007; 13: 73–97
Donnelly LE, Barnes PJ. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol Sci 2006; 27: 546–53
Eder W, Ege MJ, von ME. The asthma epidemic. N Engl J Med 2006; 355: 2226–35
Wood AM, Stockley RA. The genetics of chronic obstructive pulmonary disease. Respir Res 2006; 7: 130
Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol 2007; 119: 1055–62
Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 1991; 67: 1033–6
Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996; 272: 60–6
Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994; 76: 301–14
Diacovo TG, Puri KD, Warnock RA, et al. Platelet-mediated lymphocyte delivery to high endothelial venules. Science 1996; 273: 252–5
McEver RP. Leukocyte-endothelial cell interactions. Curr Opin Cell Biol 1992; 4: 840–9
Berlin C, Bargatze RF, Campbell JJ, et al. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 1995; 80: 413–22
von Andrian UH, Mackay CR. T-cell function and migration: two sides of the same coin. N Engl J Med 2000; 343: 1020–34
Hidalgo A, Peired AJ, Wild MK, et al. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 2007; 26: 477–89
Xu B, Wagner N, Pham LN, et al. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4betal integrin/ VCAM-1, and LFA-1 adhesion pathways. J Exp Med 2003; 197: 1255–67
Doerschuk CM. Leukocyte trafficking in alveoli and airway passages. Respir Res 2000; 1: 136–40
Galkina E, Thatte J, Dabak V, et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest 2005; 115: 3473–83
Kuebler WM. Selectins revisited: the emerging role of platelets in inflammatory lung disease. J Clin Invest 2006; 116: 3106–8
Sperandio M, Smith ML, Forlow SB, et al. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med 2003; 197: 1355–63
Zarbock A, Polanowska-Grabowska RK, Leyv K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev 2007; 21: 99–111
Ben Horin S, Bank I. The role of very late antigen-1 in immune-mediated inflammation. Clin Immunol 2004; 113: 119–29
Davis LS, Oppenheimer Marks N, Bednarczyk JL, et al. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol 1990; 145: 785–93
Geppert TD, Lipsky PE. Activation of T lymphocytes by immobilized monoclonal antibodies to CD3: regulatory influences of monoclonal antibodies to additional T cell surface determinants. J Clin Invest 1988; 81: 1497–505
Kuebler WM, Kuhnle GE, Groh J, et al. Leukocyte kinetics in pulmonary microcirculation: intravital fluorescence microscopic study. J Appl Physiol 1994; 76: 65–71
Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest 2006; 116: 3211–9
Tosi MF, Stark JM, Hamedani A, et al. Intercellular adhesion molecule-1 (ICAM-l)-dependent and ICAM-1-independent adhesive interactions between polymorphonuclear leukocytes and human airway epithelial cells infected with parainfluenza virus type 2. J Immunol 1992; 149: 3345–9
Smyth LJ, Kirby JA, Cunningham AC. Role of the mucosal integrin alpha(E)(CD103)beta(7) in tissue-restricted cytotoxicity. Clin Exp Immunol 2007
McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol 2002; 14: 581–6
Ley K. The role of selectins in inflammation and disease. Trends Mol Med 2003; 9: 263–8
Bevilacqua MP, Nelson RM. Selectins. J Clin Invest 1993; 91: 379–87
Phan UT, Waldron TT, Springer TA. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol 2006; 7: 883–9
Lou J, Yago T, Klopocki AG, et al. Flow-enhanced adhesion regulated by a selectin interdomain hinge. J Cell Biol 2006; 174: 1107–17
Thomas W. For catch bonds, it all hinges on the interdomain region. J Cell Biol 2006; 174: 911–3
Zhu C, McEver RP. Catch bonds: physical models and biological functions. Mol Cell Biomech 2005; 2: 91–104
Somers WS, Tang J, Shaw GD, et al. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell 2000; 103: 467–79
Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med 2005; 9: 255–66
Doukas J, Pober JS. IFN-gamma enhances endothelial activation induced by tumor necrosis factor but not IL-1. J Immunol 1990; 145: 1727–33
Bevilacqua MP, Pober JS, Mendrick DL, et al. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A 1987; 84: 9238–42
Zeuke S, Ulmer AJ, Kusumoto S, et al. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc Res 2002; 56: 126–34
McEver RP, Beckstead JH, Moore KL, et al. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest 1989; 84: 92–9
Hattori R, Hamilton KK, Fugate RD, et al. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem 1989; 264: 7768–71
Foxall C, Watson SR, Dowbenko D, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl lewisX oligosaccharide. J Cell Biol 1992; 117: 895–902
Moore KL, Stults NL, Diaz S, et al. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol 1992; 118: 445–56
Wilkins PP, Moore KL, McEver RP, et al. Tyrosine sulfation of P-selectin glycoprotein ligand-1 is required for high affinity binding to P-selectin. J Biol Chem 1995; 270: 22677–80
Imai Y, Lasky LA, Rosen SD. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature 1993; 361: 555–7
Bruehl RE, Bertozzi CR, Rosen SD. Minimal sulfated carbohydrates for recognition by L-selectin and the MECA-79 antibody. J Biol Chem 2000; 275: 32642–8
Uchimura K, Rosen SD. Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol 2006; 27: 559–65
Kawashima H. Roles of sulfated glycans in lymphocyte homing. Biol Pharm Bull 2006; 29: 2343–9
Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol 2004; 4: 325–35
Becker DJ, Lowe JB. Leukocyte adhesion deficiency type II. Biochim Biophys Acta 1999; 1455: 193–204
Bernimoulin MP, Zeng XL, Abbal C, et al. Molecular basis of leukocyte rolling on PSGL-1: predominant role of core-2 O-glycans and of tyrosine sulfate residue 51. J Biol Chem 2003; 278: 37–47
Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1994; 1: 247–60
Abraham WM, Ahmed A, Sabater JR, et al. Selectin blockade prevents antigen-induced late bronchial responses and airway hyperresponsiveness in allergic sheep. Am J Respir Crit Care Med 1999; 159: 1205–14
Fiscus LC, Van HJ, Steeber DA, et al. L-Selectin is required for the development of airway hyperresponsiveness but not airway inflammation in a murine model of asthma. J Allergy Clin Immunol 2001; 107: 1019–24
Bienenstock J, McDermott MR. Bronchus- and nasal-associated lymphoid tissues. Immunol Rev 2005; 206: 22–31
Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004; 10: 927–34
Rangel-Moreno J, Hartson L, Navarro C, et al. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 2006; 116: 3183–94
Rosen SD, Tsay D, Singer MS, et al. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am J Pathol 2005; 166: 935–44
Mayadas TN, Johnson RC, Rayburn H, et al. Leukocyte rolling and extravasation are severely compromised in P selection-deficient mice. Cell 1993; 74: 541–54
Diacovo TG, Roth SJ, Buccola JM, et al. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the β2-integrin CDllb/CD18. Blood 1996; 88: 146–57
Broide DH, Humber D, Sullivan S, et al. Inhibition of eosinophil rolling and recruitment in P-selectin- and intracellular adhesion molecule-1-deficient mice. Blood 1998; 91: 2847–56
Broide DH, Sullivan S, Gifford T, et al. Inhibition of pulmonary eosinophilia in P-selectin- and ICAM-1-deficient mice. Am J Respir Cell Mol Biol 1998; 18: 218–25
De Sanctis GT, Wolyniec WW, Green FH, et al. Reduction of allergic airway responses in P-selectin-deficient mice. J Appl Physiol 1997; 83: 681–7
Lukacs NW, John A, Berlin A, et al. E- and P-selectins are essential for the development of cockroach allergen-induced airway responses. J Immunol 2002; 169: 2120–5
Pitchford SC, Momi S, Giannini S, et al. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood 2005; 105: 2074–81
Gundel RH, Wegner CD, Torcellini CA, et al. Endothelial leukocyte adhesion molecule-1 mediates antigen-induced acute airway inflammation and late-phase airway obstruction in monkeys. J Clin Invest 1991; 88: 1407–11
Bock D, Philipp S, Wolff G. Therapeutic potential of selectin antagonists in psoriasis. Expert Opin Investig Drugs 2006; 15: 963–79
Kusaka M, Zandi-Nejad K, Kato S, et al. Exploitation of the continuum between early ischemia/reperfusion injury and host alloresponsiveness: indefinite kidney allograft survival by treatment with a soluble P-selectin ligand and low-dose cyclosporine in combination. Transplantation 1999; 67: 1255–61
Lefer DJ, Flynn DM, Phillips ML, et al. A novel sialyl LewisX analog attenuates neutrophil accumulation and myocardial necrosis after ischemia and reperfusion. Circulation 1994; 90: 2390–401
Dulkanchainun TS, Goss JA, Imagawa DK, et al. Reduction of hepatic ischemia/ reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg 1998; 227: 832–40
Hayward R, Campbell B, Shin YK, et al. Recombinant soluble P-selectin glycoprotein ligand-1 protects against myocardial ischemic reperfusion injury in cats. Cardiovasc Res 1999; 41: 65–76
Silver MJ, Sutton JM, Hook S, et al. Adjunctive selectin blockade successfully reduces infarct size beyond thrombolysis in the electrolytic canine coronary artery model. Circulation 1995; 92: 492–9
Kumar A, Villani MP, Patel UK, et al. Recombinant soluble form of PSGL-1 accelerates thrombolysis and prevents reocclusion in a porcine model. Circulation 1999; 99: 1363–9
IDdb3 database [online]. Available from URL: http://www.iddb3.com/ [Accessed 2007 Apr 30]
Hicks AE, Nolan SL, Ridger VC, et al. Recombinant P-selectin glycoprotein ligand-1 directly inhibits leukocyte rolling by all 3 selectins in vivo: complete inhibition of rolling is not required for anti-inflammatory effect. Blood 2003; 101: 3249–56
Hirata T, Furukawa Y, Yang BG, et al. Human P-selectin glycoprotein ligand-1 (PSGL-1) interacts with the skin-associated chemokine CCL27 via sulfated tyrosines at the PSGL-1 amino terminus. J Biol Chem 2004; 279: 51775–82
Bhushan M, Bleiker TO, Ballsdon AE, et al. Anti-E-selectin is ineffective in the treatment of psoriasis: a randomized trial. Br J Dermatol 2002; 146: 824–31
Ley K, Bullard DC, Arbones ML, et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med 1995; 181: 669–75
Keramidaris E, Merson TD, Steeber DA, et al. L-selectin and intercellular adhesion molecule 1 mediate lymphocyte migration to the inflamed airway/lung during an allergic inflammatory response in an animal model of asthma. J Allergy Clin Immunol 2001; 107: 734–8
Seekamp A, van GM, Dhondt E, et al. The effect of anti-L-selectin (aselizumab) in multiple traumatized patients: results of a phase II clinical trial. Crit Care Med 2004; 32: 2021–8
Friedrich M, Philipp S, Rincic M, et al. Anti-L-selectin therapy is not effective in psoriasis: a randomized trial [abstract 198]. J Invest Dermatol 2005; 125Suppl. 1: A34
Schermerhorn ML, Nelson DP, Blume ED, et al. Sialyl LewisX oligosaccharide preserves myocardial and endothelial function during cardioplegic ischemia. Ann Thorac Surg 2000; 70: 890–4
Schermerhorn ML, Tofukuji M, Khoury PR, et al. Sialyl lewis oligosaccharide preserves cardiopulmonary and endothelial function after hypothermic circulatory arrest in lambs. J Thorac Cardiovasc Surg 2000; 120: 230–7
Shin’oka T, Nagashima M, Nollert G, et al. A novel sialyl Lewis X analog attenuates cerebral injury after deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg 1999; 117: 1204–11
Tofukuji M, Metais C, Collard CD, et al. Effect of sialyl Lewis(x) oligosaccharide on myocardial and cerebral injury in the pig. Ann Thorac Surg 1999; 67: 112–9
Schmid RA, Yamashita M, Boasquevisque CH, et al. Carbohydrate selectin inhibitor CY-1503 reduces neutrophil migration and reperfusion injury in canine pulmonary allografts. J Heart Lung Transplant 1997; 16: 1054–61
Birnbaum Y, Patterson M, Kloner RA. The effect of CY1503, a sialyl Lewisx analog blocker of the selectin adhesion molecules, on infarct size and “no-reflow” in the rabbit model of acute myocardial infarction/reperfusion. J Mol Cell Cardiol 1997; 29: 2013–25
Zhang RL, Chopp M, Zhang ZG, et al. E-selectin in focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab 1996; 16: 1126–36
Gill EA, Kong Y, Horwitz LD. An oligosaccharide sialyl-Lewis(x) analogue does not reduce myocardial infarct size after ischemia and reperfusion in dogs. Circulation 1996; 94: 542–6
Misawa K, Toledo-Pereyra LH, Phillips ML, et al. Role of sialyl Lewis(x) in total hepatic ischemia and reperfusion. J Am Coll Surg 1996; 182: 251–6
Kerr KM, Auger WR, Marsh JJ, et al. The use of cylexin (CY-1503) in prevention of reperfusion lung injury in patients undergoing pulmonary thromboendarterectomy. Am J Respir Crit Care Med 2000; 162: 14–20
Kaila N, Janz K, Huang A, et al. 2-(4-Chlorobenzyl)-3-hydroxy-7,8,9,10-te-trahydrobenzo[H]quinoline-4-carboxylic acid (PSI-697): identification of a clinical candidate from the quinoline salicylic acid series of P-selectin antagonists. J Med Chem 2007; 50: 40–64
Myers Jr DD, Wrobleski SK, Longo C, et al. Resolution of venous thrombosis using a novel oral small-molecule inhibitor of P-selectin (PSI-697) without anticoagulation. Thromb Haemost 2007; 97: 400–7
Myers Jr DD, Henke PK, Bedard PW, et al. Treatment with an oral small molecule inhibitor of P selectin (PSI-697) decreases vein wall injury in a rat stenosis model of venous thrombosis. J Vasc Surg 2006; 44: 625–32
Myers Jr DD, Rectenwald JE, Bedard PW, et al. Decreased venous thrombosis with an oral inhibitor of P selectin. J Vasc Surg 2005; 42: 329–36
Friedrich M, Bock D, Philipp S, et al. Pan-selectin antagonism improves psoriasis manifestation in mice and man. Arch Dermatol Res 2006; 297: 345–51
Aydt E, Wolff G. Development of synthetic pan-selectin antagonists: a new treatment strategy for chronic inflammation in asthma. Pathobiology 2002; 70: 297–301
Kogan TP, Dupre B, Bui H, et al. Novel synthetic inhibitors of selectin-mediated cell adhesion: synthesis of l,6-bis[3-(3-carboxymethylphenyl)-4-(2-alpha-D-mannopyranosyloxy)phenyl]hexane (TBC1269). J Med Chem 1998; 41: 1099–111
Lopez-Neblina F, Toledo-Pereyra LH. Anti-ischemic effect of selectin blocker through modulation of tumor necrosis factor-alpha and interleukin-10. J Surg Res 2007; 138: 275–83
Jayle C, Milinkevitch S, Favreau F, et al. Protective role of selectin ligand inhibition in a large animal model of kidney ischemia-reperfusion injury. Kidney Int 2006; 69: 1749–55
Toledo-Pereyra LH, Lopez-Neblina F, Reuben JS, et al. Selectin inhibition modulates Akt/MAPK signaling and chemokine expression after liver ischemia-reperfusion. J Invest Surg 2004; 17: 303–13
Onai Y, Suzuki J, Nishiwaki Y, et al. Blockade of cell adhesion by a small molecule selectin antagonist attenuates myocardial ischemia/reperfusion injury. Eur J Pharmacol 2003; 481: 217–25
Nemoto T, Burne MJ, Daniels F, et al. Small molecule selectin ligand inhibition improves outcome in ischemic acute renal failure. Kidney Int 2001; 60: 2205–14
Keller VA, Pigott JD, Flint LM, et al. Age-related differences in response to neutrophil-mediated reperfusion injury in the neonatal piglet heart. Surgery 1998; 123: 294–304
Palma-Vargas JM, Toledo-Pereyra L, Dean RE, et al. Small-molecule selectin inhibitor protects against liver inflammatory response after ischemia and reperfusion. J Am Coll Surg 1997; 185: 365–72
Avila PC, Boushey HA, Wong H, et al. Effect of a single dose of the selectin inhibitor TBC1269 on early and late asthmatic responses. Clin Exp Allergy 2004; 34: 77–84
Beeh KM, Beier J, Meyer M, et al. Bimosiamose, an inhaled small-molecule pan-selectin antagonist, attenuates late asthmatic reactions following allergen challenge in mild asthmatics: a randomized, double-blind, placebo-controlled clinical cross-over-trial. Pulm Pharmacol Ther 2006; 19: 233–41
Revotar website [online]. Available from URL: http://www.revotar.com/ [Accessed 2007 Apr 30]
Kranich R, Busemann AS, Bock D, et al. Rational design of novel, potent small molecule pan-selectin antagonists. J Med Chem 2007; 50: 1101–15
Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov 2003; 2: 703–16
Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell 2002; 13: 3369–87
Hynes RO. Integrins: a family of cell surface receptors. Cell 1987; 48: 549–54
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110: 673–87
Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Ann Rev Cell Dev Biol 1995; 11: 549–99
Pribila JT, Quale AC, Mueller KL, et al. Integrins and T cell-mediated immunity. Annu Rev Immunol 2004; 22: 157–80
Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol 2005; 26: 208–14
Hynes RO. Integrins: versatility, modulation, and signalling in cell adhesion. Cell 1992; 69: 11–25
Xiao T, Takagi J, Coller BS, et al. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 2004; 432: 59–67
Michishita M, Videm V, Arnaout MA. A novel divalent cation-binding site in the A domain of the β2 integrin CR3 (Cd1 1b/CD18) is essential for ligand binding. Cell 1993; 72: 857–67
Randi AM, Hogg N. I domain of beta 2 integrin lymphocyte function-associated antigen-1 contains a binding site for ligand intercellular adhesion molecule-1. J Biol Chem 1994; 269: 12395–8
Mould AP, Humphries MJ. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr Opin Cell Biol 2004; 16: 544–51
Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol 2006; 18: 579–86
Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol 2007; 25: 619–47
Calderwood DA, Zent R, Grant R, et al. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J Biol Chem 1999; 274: 28071–4
Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 2003; 301: 1720–5
Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol 2005; 21: 381–410
Shimaoka M, Xiao T, Liu JH, et al. Structures of the alphaL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 2003; 112: 99–111
Liu S, Rose DM, Han J, et al. Alpha4 integrins in cardiovascular development and diseases. Trends Cardiovasc Med 2000; 10: 253–7
Bochner BS, Luscinskas FW, Gimbrone Jr MA, et al. Adhesion of human baso-phils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med 1991; 173: 1553–7
Hemler ME, Huang C, Takada Y, et al. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem 1987; 262: 11478–85
Gismondi A, Morrone S, Humphries MJ, et al. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol 1991; 146: 384–92
Elices MJ, Osborn L, Takada Y, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4/fibronectin binding site. Cell 1990; 60: 577–84
Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989; 59: 1203–11
Wayner EA, Garcia-Pardo A, Humphries MJ, et al. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol 1989; 109: 1321–30
Humphries JD, Humphries MJ. CD14 is a ligand for the integrin alpha4betal. FEBS Lett 2007; 581: 757–63
Cunningham SA, Rodriguez JM, Arrate MP, et al. JAM2 interacts with alpha4betal: facilitation by JAM3. J Biol Chem 2002; 277: 27589–92
Bridges LC, Tani PH, Hanson KR, et al. The lymphocyte metalloprotease MDC-L (ADAM 28) is a ligand for the integrin alpha4betal. J Biol Chem 2002; 277: 3784–92
Grabovsky V, Feigelson S, Chen C, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med 2000; 192: 495–506
Shimizu Y, Seventer GAV, Horgan KJ, et al. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol 1990; 145: 59–67
Rose DM, Grabovsky V, Alon R, et al. The affinity of integrin alpha(4)beta(l) governs lymphocyte migration. J Immunol 2001; 167: 2824–30
Bednarczyk JL, Mclntyre BW. A monoclonal antibody to VLA-4 α-chain (CDw49d) induces homotypic lymphocyte aggregation. J Immunol 1990; 144: 777–84
Matsunaga T, Takemoto N, Sato T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 2003; 9: 1158–65
Abraham WM, Sielczak MW, Ahmed A, et al. Alpha 4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J Clin Invest 1994; 93: 776–87
Henderson WR, Chi EY, Albert RK, et al. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest 1997; 100: 3083–92
Metzger WJ. Therapeutic approaches to asthma based on VLA-4 integrin and its counter receptors. Springer Semin Immunopathol 1995; 16: 467–78
Hojo M, Maghni K, Issekutz TB, et al. Involvement of alpha-4 integrins in allergic airway responses and mast cell degranulation in vivo. Am J Respir Crit Care Med 1998; 158: 1127–33
Pretolani M, Ruffle C, Lapa e Silva JR, et al. Antibody to very late activation antigen 4 prevents antigen-induced bronchial hyperreactivity and cellular infiltration in the guinea pig airways. J Exp Med 1994; 180: 795–805
Palmer EL, Ruegg C, Ferrando R, et al. Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J Cell Biol 1993; 123: 1289–97
Taooka Y, Chen J, Yednock T, et al. The integrin alpha9betal mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol 1999; 145: 413–20
Yokosaki Y, Monis H, Chen J, et al. Differential effects of the integrins alpha9betal, alphavbeta3, and alphavbeta6 on cell proliferative responses to tenascin: roles of the beta subunit extracellular and cytoplasmic domains. J Biol Chem 1996; 271: 24144–50
Smith LL, Cheung H-K, Ling LE, et al. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by α9β1 integrin. J Biol Chem 1996; 271: 28485–91
Eto K, Puzon-McLaughlin W, Sheppard D, et al. RGD-independent binding of integrin alpha9betal to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem 2000; 275: 34922–30
Vlahakis NE, Young BA, Atakilit A, et al. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9betal. J Biol Chem 2005; 280: 4544–52
Vlahakis NE, Young BA, Atakilit A, et al. Integrin alpha 9beta 1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A induced angiogenesis. J Biol Chem 2007
Mishima K, Watabe T, Saito A, et al. Proxl induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell 2007; 18: 1421–9
Huang XZ, Wu JF, Ferrando R, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9betal. Mol Cell Biol 2000; 20: 5208–15
Chen C, Huang X, Atakilit A, et al. The integrin alpha9beta1 contributes to granulopoiesis by enhancing granulocyte colony-stimulating factor receptor signaling. Immunity 2006; 25: 895–906
Hemler ME, Jacobson JG, Brenner MB, et al. VLA-1: a T cell surface antigen which defines a novel late stage of human T cell activation. Eur J Immunol 1985; 15: 502–8
Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol 2000; 19: 319–23
Racine-Samson L, Rockey DC, Bissell DM. The role of alpha1betal integrin in wound contraction: a quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem 1997; 272: 30911–7
Pozzi A, Wary KK, Giancotti FG, et al. Integrin alpha1betal mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol 1998; 142: 587–94
Farndale RW, Sixma JJ, Barnes MJ, et al. The role of collagen in thrombosis and hemostasis. J Thromb Haemost 2004; 2: 561–73
Rubio MA, Sotillos M, Jochems G, et al. Monocyte activation: rapid induction of alpha 1/beta 1 (VLA-1) integrin expression by lipopolysaccharide and inter-feron-gamma. Eur J Immunol 1995; 25: 2701–5
Lundberg S, Lindholm J, Lindbom L, et al. Integrin alpha2betal regulates neutrophil recruitment and inflammatory activity in experimental colitis in mice. Inflamm Bowel Dis 2006; 12: 172–7
Corrigan CJ, Hartneil A, Kay AB. T lymphocyte activation in acute severe asthma. Lancet 1988; 1: 1129–32
Bazan-Socha S, Bukiej A, Pulka G, et al. Increased expression of collagen receptors: alpha1betal and alpha2betal integrins on blood eosinophils in bronchial asthma. Clin Exp Allergy 2006; 36: 1184–91
Calderwood DA, Tuckwell DS, Eble J, et al. The integrin alpha1 A-domain is a ligand binding site for collagens and laminin. J Biol Chem 1997; 272: 12311–7
Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J Histochem Cytochem 2000; 48: 1291–306
Schwarzbauer J. Basement membranes: putting up the barriers. Curr Biol 1999; 9: R242–4
Tulla M, Pentikainen OT, Viitasalo T, et al. Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 101 domains. J Biol Chem 2001; 276: 48206–12
Abraham WM, Ahmed A, Serebriakov I, et al. A monoclonal antibody to alpha1betal blocks antigen-induced airway responses in sheep. Am J Respir Crit Care Med 2004; 169: 97–104
Vanderslice P, Woodside DG. Integrin antagonists as therapeutics for inflammatory diseases. Expert Opin Investig Drugs 2006; 15: 1235–55
Leckie MJ, Jenkins GR, Khan J, et al. Sputum T lymphocytes in asthma, COPD and healthy subjects have the phenotype of activated intraepithelial T cells (CD69+ CD103+). Thorax 2003; 58: 23–9
Pauls K, Schon M, Kubitza RC, et al. Role of integrin alphaE(CD103)beta7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J Invest Dermatol 2001; 117: 569–75
Reimann J, Rudolphi A. Co-expression of CD8 alpha in CD4+ T cell receptor alpha beta + T cells migrating into the murine small intestine epithelial layer. Eur J Immunol 1995; 25: 1580–8
Pribila JT, Itano AA, Mueller KL, et al. The alpha 1 beta 1 and alpha E beta 7 integrins define a subset of dendritic cells in peripheral lymph nodes with unique adhesive and antigen uptake properties. J Immunol 2004; 172: 282–91
Woolf E, Brenner O, Goldenberg D, et al. Runx3 regulates dendritic epidermal T cell development. Dev Biol 2007; 303: 703–14
Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med 2005; 202: 1063–73
Beaty SR, Rose Jr CE, Sung SS. Diverse and potent chemokine production by lung CD11b high dendritic cells in homeostasis and in allergic lung inflammation. J Immunol 2007; 178: 1882–95
Sung SS, Fu SM, Rose Jr CE, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 2006; 176: 2161–72
Taraszka KS, Higgins JM, Tan K, et al. Molecular basis for leukocyte integrin alpha(E)beta(7) adhesion to epithelial (E)-cadherin. J Exp Med 2000; 191: 1555–67
Suffia I, Reckling SK, Salay G, et al. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol 2005; 174: 5444–55
Allez M, Brimnes J, Shao L, et al. Activation of a unique population of CD8(+) T cells by intestinal epithelial cells. Ann N Y Acad Sci 2004; 1029: 22–35
Allez M, Brimnes J, Dotan I, et al. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology 2002; 123: 1516–26
El-Asady R, Yuan R, Liu K, et al. TGF-ta-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med 2005; 201: 1647–57
Hadley G. Role of integrin CD103 in promoting destruction of renal allografts by CD8 T cells. Am J Transplant 2004; 4: 1026–32
Glader PS, Lofdahl CG, von Wachenfeldt KA. alphaEbeta7 expression on CD8+ T-cells in COPD BAL fluid and on TGF-beta stimulated T-cells in vitro. Lung 2005; 183: 123–38
Le FA, Jalil A, Vergnon I, et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med 2007; 204: 59–70
Crowley CA, Curnutte JT, Rosin RE, et al. An inherited abnormality of neutrophil adhesion: its genetic transmission and its association with a missing protein. N Engl J Med 1980; 302: 1163–8
Arnaout MA, Pitt J, Cohen HJ, et al. Deficiency of a granulocyte-membrane glycoprotein (gp l50) in a boy with recurrent bacterial infections. N Engl J Med 1982; 306: 693–9
Springer TA, Thompson WS, Miller LJ, et al. Inherited deficiency of the Mac-1, LFA-1, pl50,95 glycoprotein family and its molecular basis. J Exp Med 1984; 160: 1901–18
Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and pl50-95 glycoproteins. Annu Rev Med 1987; 38: 175–83
Shimaoka M, Springer TA. Therapeutic antagonists and the conformational regulation of the beta2 integrins. Curr Top Med Chem 2004; 4: 1485–95
Dustin ML, Springer TA. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol 1988; 107: 321–31
Issekutz AC, Rowter D, Springer TA. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J Leukoc Biol 1999; 65: 117–26
Shang XZ, Issekutz AC. Contribution of CDlla/CD18, CD11b/CD18, ICAM-1 (CD54) and -2 (CD102) to human monocyte migration through endothelium and connective tissue fibroblast barriers. Eur J Immunol 1998; 28: 1970–9
Wacholtz MC, Patel SS, Lipsky PE. Leukocyte function-associated antigen 1 is an activation molecule for human T cells. J Exp Med 1989; 170: 431–48
Berlin-Rufenach C, Otto F, Mathies M, et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med 1999; 189: 1467–78
Monks CR, Freiberg BA, Kupfer H, et al. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998; 395: 82–6
Carrasco YR, Fleire SJ, Cameron T, et al. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 2004; 20: 589–99
Davignon D, Martz E, Reynolds T, et al. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci U S A 1981; 78: 4535–9
Basit A, Reutershan J, Morris MA, et al. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am J Physiol Lung Cell Mol Physiol 2006; 291: L200–7
Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood 1994; 84: 2068–101
Santoso S, Sachs UJ, Kroll H, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med 2002; 196: 679–91
Phillipson M, Heit B, Colarusso P, et al. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 2006; 203: 2569–75
Staunton DE, Lupher ML, Liddington R, et al. Targeting integrin structure and function in disease. Adv Immunol 2006; 91: 111–57
Harlan JM, Winn RK. Leukocyte-endothelial interactions: clinical trials of anti-adhesion therapy. Crit Care Med 2002; 30(5 Suppl.): S214–9
Winn R, Vedder N, Ramamoorthy C, et al. Endothelial and leukocyte adhesion molecules in inflammation and disease. Blood Coagul Fibrinolysis 1998; 9Suppl. 2: S17–23
Winn RK, Sharar SR, Vedder NB, et al. Leukocyte and endothelial adhesion molecules in ischaemia/reperfusion injuries. Ciba Found Symp 1995; 189: 63–71
Edwards CP, Champe M, Gonzalez T, et al. Identification of amino acids in the CD11a I-domain important for binding of the leukocyte function-associated antigen-1 (LFA-1) to intercellular adhesion molecule-1 (ICAM-1). J Biol Chem 1995; 270: 12635–40
Raptiva prescribing information [online]. Available from URL: http://www.gene.com/gene/common/inc/pi/raptiva.jsp [Accessed 2007 Apr 30]
Werther WA, Gonzalez TN, O’Connor SJ, et al. Humanization of an anti-lymphocyte function-associated antigen (LFA)-1 monoclonal antibody and reengineering of the humanized antibody for binding to rhesus LFA-1. J Immunol 1996; 157: 4986–95
Champe M, McIntyre BW, Berman PW. Monoclonal antibodies that block the activity of leukocyte function-associated antigen 1 recognize three discrete epitopes in the inserted domain of CD11a. J Biol Chem 1995; 270: 1388–94
Huang L, Shimaoka M, Rondon IJ, et al. Identification and characterization of a human monoclonal antagonistic antibody AL-57 that preferentially binds the high-affinity form of lymphocyte function-associated antigen-1. J Leukoc Biol 2006; 80: 905–14
Lowes MA, Chamian F, Abello MV, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci U S A 2005; 102: 19057–62
Nguyen VA, Ebner S, Furhapter C, et al. Adhesion of dendritic cells derived from CD34+ progenitors to resting human dermal microvascular endothelial cells is down-regulated upon maturation and partially depends on CD11a-CD18, CD11b-CD18 and CD36. Eur J Immunol 2002; 32: 3638–50
Gauvreau GM, Becker AB, Boulet LP, et al. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol 2003; 112: 331–8
Potin D, Launay M, Monatlik F, et al. Discovery and development of 5-[(5S,9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-l-methyl-2,4-dioxo-l, 3,7-triazaspiro[4.4]non-7-yl-methyl]-3-thiophenecarboxylic acid (BMS-587101): a small molecule antagonist of leukocyte function associated antigen-1. J Med Chem 2006; 49: 6946–9
Chowdari NS, Barbas III CF. Total synthesis of LFA-1 antagonist BIRT-377 via organocatalytic asymmetric construction of a quaternary Stereocenter. Org Lett 2005; 7: 867–70
Gadek TR, Burdick DJ, McDowell RS, et al. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science 2002; 295: 1086–9
Kallen J, Welzenbach K, Ramage P, et al. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol 1999; 292: 1–9
Last-Barney K, Davidson W, Cardozo M, et al. Binding site elucidation of hydantoin-based antagonists of LFA-1 using multidisciplinary technologies: evidence for the allosteric inhibition of a protein-protein interaction. J Am Chem Soc 2001; 123: 5643–50
Shoda M, Harada T, Yano K, et al. Virtual screening leads to the discovery of an effective antagonist of lymphocyte function-associated antigen-1. Chem Med Chem 2007; 2: 515–21
Vanderslice P, Biediger RJ, Woodside DG, et al. Development of cell adhesion molecule antagonists as therapeutics for asthma and COPD. Pulm Pharmacol Ther 2004; 17: 1–10
Hijazi Y, Welker H, Dorr AE, et al. Pharmacokinetics, safety, and tolerability of R411, a dual alpha4betal -alpha4beta7 integrin antagonist after oral administration at single and multiple once-daily ascending doses in healthy volunteers. J Clin Pharmacol 2004; 44: 1368–78
Rames AD, Busse WW, Renzetti L, et al. R411 treatment reduces asthma exacerbations and attenuates FEV1 fall following steroid withdrawal in moderate asthma [abstract]. Eur Respir J 2005; 26Suppl. 49: P1721
Sweeney BJ, Miller RF, Harrison MJ. Progressive multifocal leucoencephalopathy. Br J Hosp Med 1993; 50: 187–92
Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006; 354: 924–33
Baker M, Shock A, Parton T, et al. Pharmacokinetic and pharmacodynamic properties of the VLA-4 inhibitor CDP323 [abstract]. Multiple Scler 2006; 12(1 Suppl.): P392
Acknowledgments
The authors are employees of Encysive Pharmaceuticals, a company involved in the discovery and development of drugs for a number of cardiovascular and inflammatory diseases, including cell adhesion antagonists for the treatment of asthma. No other sources of funding were used to assist in the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woodside, D.G., Vanderslice, P. Cell Adhesion Antagonists. BioDrugs 22, 85–100 (2008). https://doi.org/10.2165/00063030-200822020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200822020-00002