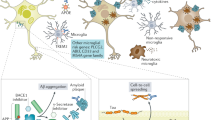
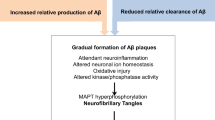
Abstract
The tremendous advances in transgene animal technology, especially in the area of Alzheimer’s disease, have not resulted in a significantly better success rate for drugs entering clinical development. Despite substantial increases in research and development budgets, the number of approved drugs in general has not increased, leading to the so-called innovation gap. While animal models have been very useful in documenting the possible pathological mechanisms in many CNS diseases, they are not very predictive in the area of drug development.
This paper reports on a number of under-appreciated fundamental differences between animal models and human patients in the context of drug discovery with special emphasis on Alzheimer’s disease and schizophrenia, such as different affinities of the same drug for human versus rodent target subtypes and the absence of many functional genotypes in animal models. I also offer a number of possible solutions to bridge the translational disconnect and improve the predictability of preclinical models, such as more emphasis on good-quality translational studies, more pre-competitive information sharing and the embracing of multi-target pharmacology strategies.
Re-engineering the process for drug discovery and development, in a similar way to other more successful industries, is another possible but disrupting solution to the growing innovation gap. This includes the development of hybrid computational models, based upon documented preclinical physiology and pharmacology, but populated and validated with clinical data from actual patients.



Similar content being viewed by others
References
Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 2004 Aug; 3(8): 711–5
Alzheimer Research Forum. Live discussion: mice on trial? Issues in the design of drug studies [online]. Available from URL: http://www.alzforum.org/res/for/journal/detail.asp?liveID=168 [Accessed 2009 Jul 23]
Akhtar AZ, Pippin JJ, Sandusky CB. Animal models in spinal cord injury: a review. Rev Neurosci 2008; 19(1): 47–60
Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 1998 Aug 28; 281(5381): 1349–52
Ryman D, Lamb BT. Genetic and environmental modifiers of Alzheimer’s disease phenotypes in the mouse. Curr Alzheimer Res 2006 Dec; 3(5): 465–73
Lassalle JM, Halley H, Daumas S, et al. Effects of the genetic background on cognitive performances of TG2576 mice. Behav Brain Res 2008 Aug 5; 191(1): 104–10
Gloriam DE, Fredriksson R, Schiöth HB. The G protein-coupled receptor subset of the rat genome. BMC Genomics 2007 Sep 25; 8: 338–45
PDSP. NIMH Psychoactive Drug Screening Program [online]. Available from URL: http://pdsp.med.unc.edu/ [Accessed 2009 Jul 23]
Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress 2004 Jun; 7(2): 131–43
Muly EC, Maddox M, Smith Y. Distribution of mGluR1-alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol 2003; 467(4): 521–35
Tooney PA, Au GG, Chahl LA. Localisation of tachykinin NK1 and NK3 receptors in the human prefrontal and visual cortex. Neurosci Lett 2000; 283(3): 185–8
Whitty CJ, Paul MA, Bannon MJ. Neurokinin receptor mRNA localization in human midbrain dopamine neurons. J Comp Neurol 1997; 382(3): 394–400
Marazziti D, Betti L, Giannaccini G, et al. Distribution of [3H]GR65630 binding in human brain postmortem. Neurochem Res 2001 Mar; 26(3): 187–90
Hewlett WA, Fridman S, Trivedi BL, et al. Characterization of desamino-5-[125I]iodo-3-methoxy-zacopride ([125I]MI-ZAC) binding to 5-HT3 receptors in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry 1998 Feb; 22(2): 397–410
Hirst WD, Abrahamsen B, Blaney FE, et al. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol 2003 Dec; 64(6): 1295–308
Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993 Aug; 43(8): 1467–72
Raffai RL, Dong LM, Farese Jr RV, et al. Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proc Natl Acad Sci U S A 2001 Sep 25; 98(20): 11587–91
Loring JF, Paszty C, Rose A, et al. Rational design of an animal model for Alzheimer’s disease: introduction of multiple human genomic transgenes to reproduce AD pathology in a rodent. Neurobiol Aging 1996 Mar–Apr; 17(2): 173–82
Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex 2007 Sep; 17Suppl. 1: i171–81
Bertolino A, Caforio G, Blasi G, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry 2004 Oct; 161(10): 1798–805
Salminen M, Lundström K, Tilgmann C, et al. Molecular cloning and characterization of rat liver catechol-O-methyltransferase. Gene 1990 Sep 14; 93(2): 241–7
Babovic D, O’Tuathaigh CM, O’Sullivan GJ, et al. Exploratory and habituation phenotype of heterozygous and homozygous COMT knockout mice. Behav Brain Res 2007 Nov 2; 183(2): 236–9
Meno-Tetang GM, Lowe PJ. On the prediction of the human response: a recycled mechanistic pharmacokinetic/ pharmacodynamic approach. Basic Clin Pharmacol Toxicol 2005 Mar; 96(3): 182–92
Katoh M, Tateno C, Yoshizato K, et al. Chimeric mice with humanized liver. Toxicology 2008 Apr 3; 246(1): 9–17
Kapur S, VanderSpek SC, Brownlee BA, et al. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther 2003 May; 305(2): 625–31
Ikonomovic MD, Abrahamson EE, Isanski BA, et al. Superior frontal cortex cholinergic axon density in mild cognitive impairment and early Alzheimer disease. Arch Neurol 2007 Sep; 64(9): 1312–7
Raschetti R, Albanese E, Vanacore N, et al. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med 2007 Nov 27; 4(11): e338
Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 2007 Nov; 130 Pt 11: 2837–44
Korczyn A. The amyloid cascade hypothesis. Alzheimers Dement 2008; 4: 176–8
Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 2008; 29(10): 1456–65
Carpenter WT, Koenig JI. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology 2008 Aug; 33(9): 2061–79
Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A 2000 Jul 5; 97(14): 8104–9
Bredeloux P, Dubuc I, Costentin J. Comparisons between bupropion and dexamphetamine in a range of in vivo tests exploring dopaminergic transmission. Br J Pharmacol 2007 Mar; 150(6): 711–9
Boileau I, Dagher A, Leyton M, et al. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci 2007 Apr 11; 27(15): 3998–4003
Suri RE. TD models of reward predictive responses in dopamine neurons. Neural Netw 2002 Jun–Jul; 15(4–6): 523–33
Wanjerkhede SM, Bapi RS. Modeling the sub-cellular signaling pathways involved in reinforcement learning at the striatum. Prog Brain Res 2008; 168: 193–206
Roberts WA, Feeney MC, Macpherson K, et al. Episodic-like memory in rats: is it based on when or how long ago? Science 2008 Apr 4; 320(5872): 113–5
Skelley SL, Goldberg TE, Egan MF, et al. Verbal and visual memory: characterizing the clinical and intermediate phenotype in schizophrenia. Schizophr Res 2008 Oct; 105(1–3): 78–85
Howard AR. Further validation studies of the Wechsler Memory Scale. Clin Psychol 1954 Apr; 10(2): 164–7
Traykov L, Raoux N, Latour F, et al. Executive functions deficit in mild cognitive impairment. Cogn Behav Neurol 2007 Dec; 20(4): 219–24
Carlson JM, Doyle J. Highly optimized tolerance: robustness and design in complex systems. Phys Rev Lett 2000 Mar 13; 84(11): 2529–32
Nayak S, Salim S, Luan D, et al. A test of highly optimized tolerance reveals fragile cell-cycle mechanisms are molecular targets in clinical cancer trials. PLoS One 2008 Apr 23; 3(4): e2016
Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol 2008 Jul 1; 6(7): e159
Sambataro F, Murty VP, Callicott JH, et al. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. Epub 2008 Jul 30
Winder R, Cortes CR, Reggia JA, et al. Functional connectivity in fMRI: modeling approach for estimation and for relating to local circuits. Neuroimage 2007 Feb 1; 34(3): 1093–107
Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 2000 Apr; 157(4): 514–20
Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003 Jun; 60(6): 553–64
Stroup T. Heterogeneity of treatment effects in schizophrenia. Am J Med 2007 Apr; 120(4 Suppl. 1): S26–31
Wong EH, Nikam SS, Shahid M. Multi- and single-target agents for major psychiatric diseases: therapeutic opportunities and challenges. Curr Opin Investig Drugs 2008 Jan; 9(1): 28–36
Nudelman A, Gil-Ad I, Shpaisman N, et al. A mutual prodrug ester of GABA and perphenazine exhibits anti-schizophrenic efficacy with diminished extrapyramidal effects. J Med Chem 2008 May 8; 51(9): 2858–62
ClinicalTrials.gov. Available from URL: http://www.clinicaltrials.gov/ct2/results?term=Alzheimer [Accessed 2009 Jul 23]
Cambridge Healthtech Associates. Our mission [online]. Available from URL: http://www.chacorporate.com/pages/55_our_mission.cfm [Accessed 2009 Jul 23]
Cambridge Healthtech Associates. Toxicokinetics (TK) optimization [online]. Available from URL: http://www.chacorporate.com/pages/91_toxicokinetics_tk_optimization.cfm [Accessed 2009 Jul 23]
Montague PR, King-Casas B, Cohen JD. Imaging valuation models in human choice. Annu Rev Neurosci 2006; 29: 417–48
Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nat Neurosci 2006 Sep; 9(9): 1177–85
Mitchell TM, Shinkareva SV, Carlson A, et al. Predicting human brain activity associated with the meanings of nouns. Science 2008 May 30; 320(5880): 1191–5
Yoshimura M, Koenig T, Irisawa S, et al. A pharmaco-EEG study on antipsychotic drugs in healthy volunteers. Psychopharmacology (Berl) 2007 May; 191(4): 995–1004
Ahnaou A, Nayak S, Heylen A, et al. Sleep and EEG profile in neonatal hippocampal lesion model of schizophrenia. Physiol Behav 2007 Oct 22; 92(3): 461–7
Salvadore G, Cornwell BR, Colon-Rosario V, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry 2009 Feb 15; 65(4): 289–95
Holmes HM, Sachs GA, Shega JW, et al. Integrating palliative medicine into the care of persons with advanced dementia: identifying appropriate medication use. J Am Geriatr Soc 2008 Jul; 56(7): 1306–11
Geerts H. Drug evaluation: (R)-flurbiprofen an enantiomer of flurbiprofen for the treatment of Alzheimer’s disease. IDrugs 2007 Feb; 10(2): 121–33
Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer’s disease: the pivotal role of brain M1 receptors. Neurodegener Dis 2008; 5(3–4): 237–40
Menzies L, Ooi C, Kamath S, et al. Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry 2007 Feb; 64(2): 156–67
Kirschner M. The meaning of systems ioilogy. Cell 2005; 121(4): 503–4
Bangs A. Predictive biosimulation and virtual patients in pharmaceutical R and D. Stud Health Technol Inform 2005; 111: 37–42
Cappuccio A, Elishmereni M, Agur Z. Cancer immunotherapy by interleukin-21: potential treatment strategies evaluated in a mathematical model. Cancer Res 2006 Jul 15; 66(14): 7293–300
Hodgkin AL, Huxley AF. A quantitative description of membrane currents and its application to conduction and excitation in nerve. J Physiol 1952; 117(4): 500–44
De Schutter E. Why are computational neuroscience and systems biology so separate? PLoS Comput Biol 2008 May 30; 4(5): e1000078
Erdi P, Kiss T, Tóth J, et al. From systems biology to dynamical neuropharmacology: proposal for a new methodology. Syst Biol (Stevenage) 2006 Jul; 153(4): 299–308
Acknowledgements
Discussions with scientists at the Center for Neurodegenerative Disease (CNDR) laboratory at the University of Pennsylvania, in particular Kurt Brunden, Virginia Lee and John Trojanowski, are very much appreciated. The author is an employee of In Silico Biosciences, Inc., has acted as a consultant for the M. Ware Foundation, CNDR, University of Pennsylvania, and has received honoraria from Janssen Pharmaceutica. He is co-inventor and has a patent pending on mechanistic disease modelling in CNS disorders.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geerts, H. Of Mice and Men. CNS Drugs 23, 915–926 (2009). https://doi.org/10.2165/11310890-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11310890-000000000-00000