Abstract
Frontotemporal lobar degeneration (FTLD) is a clinically and pathologically heterogeneous syndrome, characterized by progressive decline in behaviour or language associated with degeneration of the frontal and anterior temporal lobes. While the seminal cases were described at the turn of the 20th century, FTLD has only recently been appreciated as a leading cause of dementia, particularly in patients presenting before the age of 65 years. Three distinct clinical variants of FTLD have been described: (i) behavioural-variant frontotemporal dementia, characterized by changes in behaviour and personality in association with frontal-predominant cortical degeneration; (ii) semantic dementia, a syndrome of progressive loss of knowledge about words and objects associated with anterior temporal neuronal loss; and (iii) progressive nonfluent aphasia, characterized by effortful language output, loss of grammar and motor speech deficits in the setting of left perisylvian cortical atrophy.
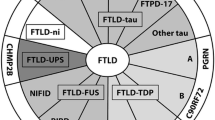
The majority of pathologies associated with FTLD clinical syndromes include either tau-positive (FTLD-TAU) or TAR DNA-binding protein 43 (TDP-43)-positive (FTLD-TDP) inclusion bodies. FTLD overlaps clinically and pathologically with the atypical parkinsonian disorders corticobasal degeneration and progressive supranuclear palsy, and with amyotrophic lateral sclerosis. The majority of familial FTLD cases are caused by mutations in the genes encoding microtubule-associated protein tau (leading to FTLD-TAU) or progranulin (leading to FTLD-TDP).
The clinical and pathological heterogeneity of FTLD poses a significant diagnostic challenge, and in vivo prediction of underlying histopathology can be significantly improved by supplementing the clinical evaluation with genetic tests and emerging biological markers.
Current pharmacotherapy for FTLD focuses on manipulating serotonergic or dopaminergic neurotransmitter systems to ameliorate behavioural or motor symptoms. However, recent advances in FTLD genetics and molecular pathology make the prospect of biologically driven, diseasespecific therapies for FTLD seem closer than ever.




Similar content being viewed by others
References
Alzheimer A. Uber eigenartige Krankheitsfalle des sparteren Alters. Psychiatr Nervenkr Z Gesamte Neurol Psychiatr 1911; 4: 356–85
Pick A. Uber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Med Wochenschr 1892; 7: 165–7
Pick A. Zur symptomatologie der linksseitigen schlafenappenatrophie. Monatsschrift Psychiatry Neurol 1904; 16: 378–88
Wilhelmsen KC. Frontotemporal dementia is on the MAPtau. Ann Neurol 1997; 41: 139–40
Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–5
Spillantini MG, Murrell JR, Goedert M, et al. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 1998; 95: 7737–41
Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–3
Gass J, Cannon A, Mackenzie IR, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 2006; 15: 2988–3001
Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442: 916–9
Cruts M, Kumar-Singh S, Van Broeckhoven C. Progranulin mutations in ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Curr Alzheimer Res 2006; 3: 485–91
Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–54
Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain 2003; 126: 2016–22
Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology 2002; 58: 1615–21
Mercy L, Hodges JR, Dawson K, et al. Incidence of earlyonset dementias in Cambridgeshire, United Kingdom. Neurology 2008; 71: 1496–9
Knopman DS, Petersen RC, Edland SD, et al. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology 2004; 62: 506–8
Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol 2005; 62: 925–30
Hodges JR, Davies R, Xuereb J, et al. Survival in frontotemporal dementia. Neurology 2003; 61: 349–54
Gislason TB, Sjogren M, Larsson L, et al. The prevalence of frontal variant frontotemporal dementia and the frontal lobe syndrome in a population based sample of 85 year olds. J Neurol Neurosurg Psychiatry 2003; 74: 867–71
Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 2002; 16: 203–12
Brunnstrom H, Gustafson L, Passant U, et al. Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Arch Gerontol Geriatr 2009; 49: 146–9
Roberson ED, Hesse JH, Rose KD, et al. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology 2005; 65: 719–25
Rascovsky K, Salmon DP, Lipton AM, et al. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology 2005; 65: 397–403
Kertesz A, Blair M, McMonagle P, et al. The diagnosis and course of frontotemporal dementia. Alzheimer Dis Assoc Disord 2007; 21: 155–63
Kertesz A, McMonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain 2005; 128: 1996–2005
Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 2004; 62: 742–8
Gustafson L. Frontal lobe degeneration of non-Alzheimer type: II. Clinical picture and differential diagnosis. Arch Gerontol Geriatr 1987; 6: 209–23
Levy ML, Miller BL, Cummings JL, et al. Alzheimer disease and frontotemporal dementias: behavioral distinctions. Arch Neurol 1996; 53: 687–90
Neary D, Snowden JS, Northen B, et al. Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry 1988; 51: 353–61
The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994; 57: 416–8
Gustafson L. Clinical picture of frontal lobe degeneration of non-Alzheimer type. Dementia 1993; 4: 143–8
Miller BL, Cummings JL, Villanueva-Meyer J, et al. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology 1991; 41: 1374–82
Miller BL, Darby A, Benson DF, et al. Aggressive, socially disruptive and antisocial behaviour associated with fronto-temporal dementia. Br J Psychiatry 1997; 170: 150–4
Mendez MF, Chen AK, Shapira JS, et al. Acquired sociopathy and frontotemporal dementia. Dement Geriatr Cogn Disord 2005; 20: 99–104
Rankin KP, Baldwin E, Pace-Savitsky C, et al. Self awareness and personality change in dementia. J Neurol Neurosurg Psychiatry 2005; 76: 632–9
Miller BL, Seeley WW, Mychack P, et al. Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology 2001; 57: 817–21
Swartz JR, Miller BL, Lesser IM, et al. Behavioural phenomenology in Alzheimer’s disease, frontotemporal dementia, and late-life depression: a retrospective analysis. J Geriatr Psychiatry Neurol 1997; 10: 67–74
Perry RJ, Miller BL. Behavior and treatment in frontotemporal dementia. Neurology 2001; 56 Suppl. 4: S46–51
Mendez MF, Shapira JS, Miller BL. Stereotypical movements and frontotemporal dementia. Mov Disord 2005; 20: 742–5
Miller BL, Darby AL, Swartz JR, et al. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia 1995; 6: 195–9
Ikeda M, Brown J, Holland AJ, et al. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2002; 73: 371–6
Woolley JD, Gorno-Tempini ML, Seeley WW, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology 2007; 69: 1424–33
Gregory CA, Serra-Mestres J, Hodges JR. Early diagnosis of the frontal variant of frontotemporal dementia: how sensitive are standard neuroimaging and neuropsychologic tests? Neuropsychiatry Neuropsychol Behav Neurol 1999; 12: 128–35
Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol 2005; 4: 771–80
Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003; 16: 211–8
Rosen HJ, Narvaez JM, Hallam B, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord 2004; 18: 202–7
Elfgren C, Brun A, Gustafson L, et al. Neuropsychological tests as discriminators between dementia of Alzheimer type and frontotemporal dementia. Int J Geriatr Psychiatry 1994; 9: 635–42
Rascovsky K, Salmon DP, Ho GJ, et al. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology 2002; 58: 1801–8
Mendez MF, Cherrier M, Perryman KM, et al. Frontotemporal dementia versus Alzheimer’s disease: differential cognitive features. Neurology 1996; 47: 1189–94
Varma AR, Snowden JS, Lloyd JJ, et al. Evaluation of the NINCDS-ADRDA criteria in the differentiation of Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry 1999; 66: 184–8
Nedjam Z, Devouche E, Dalla Barba G. Confabulation, but not executive dysfunction discriminate AD from frontotemporal dementia. Eur J Neurol 2004; 11: 728–33
Lomen-Hoerth C. Characterization of amyotrophic lateral sclerosis and frontotemporal dementia. Dement Geriatr Cogn Disord 2004; 17: 337–41
Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 2002; 59: 1077–9
Lomen-Hoerth C, Murphy J, Langmore S, et al. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 2003; 60: 1094–7
Neary D, Snowden JS, Mann DM. Cognitive change in motor neurone disease/amyotrophic lateral sclerosis (MND/ALS). J Neurol Sci 2000; 180: 15–20
Strong MJ, Lomen-Hoerth C, Caselli RJ, et al. Cognitive impairment, frontotemporal dementia, and the motor neuron diseases. Ann Neurol 2003; 54 Suppl. 5: S20–3
Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002; 58: 198–208
Boccardi M, Sabattoli F, Laakso MP, et al. Frontotemporal dementia as a neural system disease. Neurobiol Aging 2005; 26: 37–44
Schroeter ML, Raczka K, Neumann J, et al. Neural networks in frontotemporal dementia: a meta-analysis. Neurobiol Aging 2008; 29: 418–26
Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen 2007; 22: 474–88
Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 2008; 65: 249–55
Rosen HJ, Allison SC, Schauer GF, et al. Neuroanatomical correlates of behavioural disorders in dementia. Brain 2005; 128: 2612–25
Hodges JR. Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology 2001; 56: 6S–10
Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology 2005; 64: 1384–90
Snowden J, Goulding P, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 1989; 2: 167–82
Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 2003; 61: 1196–203
Hodges JR, Patterson K, Oxbury S, et al. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain 1992; 115 (Pt 6): 1783–806
Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004; 55: 335–46
Howard D, Patterson K. Pyramids and Palm trees: a test of semantic access from pictures and words. Bury St Edmunds: Thames Valley Publishing Company, 1992
Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology 2000; 54: 2277–84
Hodges JR, Patterson K, Ward R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology 1999; 13: 31–40
Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain 1997; 120: 1027–40
Mychack P, Kramer JH, Boone KB, et al. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology 2001; 56: S11–5
Gorno-Tempini ML, Rankin KP, Woolley JD, et al. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex 2004; 40: 631–44
Rankin KP, Kramer JH, Mychack P, et al. Double dissociation of social functioning in frontotemporal dementia. Neurology 2003; 60: 266–71
Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol 2005; 18: 28–36
Rosen HJ, Allison SC, Ogar JM, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology 2006; 67: 1752–6
Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006; 129: 1385–98
Kertesz A, Martinez-Lage P, Davidson W, et al. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000; 55: 1368–75
Rebeiz JJ, Kolodny EH, Richardson Jr EP. Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 1968; 18: 20–33
Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003; 18: 467–86
Belfor N, Amici S, Boxer AL, et al. Clinical and neuropsychological features of corticobasal degeneration. Mech Ageing Dev 2006; 127: 203–7
Murray R, Neumann M, Forman MS, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology 2007; 68: 1274–83
Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996; 47: 1–9
Grafman J, Litvan I, Stark M. Neuropsychological features of progressive supranuclear palsy. Brain Cogn 1995; 28: 311–20
Gorno-Tempini ML, Murray RC, Rankin KP, et al. Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase 2004; 10: 426–36
Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006; 66: 41–8
Brun A. Frontal lobe degeneration of the non-alzheimer type: I. Neuropathology. Arch Gerontol Geriatr 1987; 6: 193–208
McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 2001; 58: 1803–9
Broe M, Hodges JR, Schofield E, et al. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology 2003; 60: 1005–11
Dickson DW. Neuropathology of Pick’s disease. Neurology 2001; 56: S16–20
Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007; 114: 5–22
Onari K, Spatz H. Anatomische Beitragezur Lehre von der Pickschen umschriebene-Grosshirnriden-Atrophie (‘Picksche Krankheit’). Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr 1926; 101: 470–511
Roberson ED. Frontotemporal dementia. Curr Neurol Neurosci Rep 2006; 6: 481–9
Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 2002; 61: 935–46
Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999; 246 Suppl. 2: II6–5
Boxer AL, Geschwind MD, Belfor N, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol 2006; 63: 81–6
Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging 2008; 29: 280–9
Goedert M, Spillantini MG, Potier MC, et al. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. Embo J 1989; 8: 393–9
Munoz DG, Dickson DW, Bergeron C, et al. The neuropathology and biochemistry of frontotemporal dementia. Ann Neurol 2003; 54 Suppl. 5: S24–8
Knopman DS. Overview of dementia lacking distinctive histology: pathological designation of a progressive dementia. Dementia 1993; 4: 132–6
Lipton AM, White CL 3rd, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol (Berl) 2004; 108: 379–85
Josephs KA, Holton JL, Rossor MN, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 2004; 30: 369–73
Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci 2008; 13: 867–78
Igaz LM, Kwong LK, Xu Y, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol 2008; 173: 182–94
Neumann M, Kwong LK, Truax AC, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol 2007; 66: 177–83
Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 2007; 171: 227–40
Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 2008; 64: 60–70
Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol 2007; 114: 49–54
Weihl CC, Temiz P, Miller SE, et al. TDP-43 accumulation in inclusion body myopathy muscle suggests a common pathogenic mechanism with frontotemporal dementia. J Neurol Neurosurg Psychiatry 2008; 79: 1186–9
Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008; 319: 1668–72
Kuhnlein P, Sperfeld AD, Vanmassenhove B, et al. Two German kindreds with familial amyotrophic lateral sclerosis due to TARDBP mutations. Arch Neurol 2008; 65: 1185–9
Schumacher A, Friedrich P, Diehl-Schmid J, et al. No association of TDP-43 with sporadic frontotemporal dementia. Neurobiol Aging 2009; 30: 157–9
Gijselinck I, Sleegers K, Engelborghs S, et al. Neuronal inclusion protein TDP-43 has no primary genetic role in FTD and ALS. Neurobiol Aging 2009; 30: 1329–31
Benajiba L, Le Ber I, Camuzat A, et al. TARDBP mutations in motorneuron disease with frontotemporal lobar degeneration. Ann Neurol 2009; 65(4): 470–3
Kovacs GG, Murrell JR, Horvath S, et al. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord 2009; 24(12): 1843–7
Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 2008; 67: 555–64
Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 2008; 70: 1850–7
Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 2008; 116: 215–20
Miklossy J, Steele JC, Yu S, et al. Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/ parkinsonism-dementia complex of Guam (ALS/PDC). Acta Neuropathol 2008; 116: 625–37
Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 2007; 1184: 284–94
Josephs KA, Lin WL, Ahmed Z, et al. Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol 2008; 116: 159–67
Roeber S, Mackenzie IR, Kretzschmar HA, et al. TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol 2008; 116: 147–57
Mackenzie IR, Foti D, Woulfe J, et al. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 2008; 131: 1282–93
Neumann M, Rademakers R, Roeber S, et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 2009 Nov; 132 (Pt 11): 2922–31
Seelaar H, Klijnsma KY, de Koning I, et al. Frequency of uniquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol. Epub Nov 2009
Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell 2009 Mar 20; 136(6): 1001–4
Kwiatkowski Jr TJ, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009 Feb 27; 323(5918): 1205–8
Munoz DG, Neumann M, Kusaka H, et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol 2009 Nov; 118(5): 617–27
Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain 2003; 126: 2291–303
Cairns NJ, Grossman M, Arnold SE, et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 2004; 63: 1376–84
Neumann M, Roeber S, Kretzschmar HA, et al. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol 2009 Nov; 118(5): 605–16
Holm IE, Englund E, Mackenzie IR, et al. A reassessment of the neuropathology of frontotemporal dementia linked to chromosome 3. J Neuropathol Exp Neurol 2007; 66: 884–91
Holm IE, Isaacs AM, Mackenzie IR. Absence of FUS-immunoreactive pathology in frontotemporal dementia linked to chromosome 3 (FTD-3) caused by mutation in the CHMP2B gene. Acta Neuropathol 2009 Nov; 118(5): 719–20
Hatanpaa KJ, Bigio EH, Cairns NJ, et al. TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 2008; 67: 271–9
Mackenzie IR, Shi J, Shaw CL, et al. Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol 2006; 112: 551–9
Goldman JS, Farmer JM, Wood EM, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 2005; 65: 1817–9
Goldman JS, Adamson J, Karydas A, et al. New genes, new dilemmas: FTLD genetics and its implications for families. Am J Alzheimers Dis Other Demen 2007; 22: 507–15
van Swieten JC, Heutink P. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol 2008; 7: 965–74
Lynch T, Sano M, Marder KS, et al. Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology 1994; 44: 1878–84
Wilhelmsen K, Lynch T, Pavlou E, et al. Localization of disinhibition-dementia-parkinsonism-amyotrophy complex to 17q21-22. Am J Hum Genet 1994; 6: 1159–65
Alzheimer disease and frontotemporal dementia mutation database [online]. Available from URL: http://www.molgen.ua.ac.be/FTDMutations [Accessed 2010 Feb 9]
Pickering-Brown SM, Rollinson S, Du Plessis D, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain 2008; 131: 721–31
Pickering-Brown SM, Baker M, Nonaka T, et al. Frontotemporal dementia with Pick-type histology associated with Q336R mutation in the tau gene. Brain 2004; 127: 1415–26
Rizzini C, Goedert M, Hodges JR, et al. Tau gene mutation K257T causes a tauopathy similar to Pick’s disease. J Neuropathol Exp Neurol 2000; 59: 990–1001
Neumann M, Schulz-Schaeffer W, Crowther RA, et al. Pick’s disease associated with the novel Tau gene mutation K369I. Ann Neurol 2001; 50: 503–13
Hayashi S, Toyoshima Y, Hasegawa M, et al. Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol 2002; 51: 525–30
Hogg M, Grujic ZM, Baker M, et al. The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol (Berl) 2003; 106: 323–36
Hasegawa M, Smith MJ, Goedert M. Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 1998; 437: 207–10
D’Souza I, Poorkaj P, Hong M, et al. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci U S A 1999; 96: 5598–603
Seelaar H, Kamphorst W, Rosso SM, et al. Distinct genetic forms of frontotemporal dementia. Neurology 2008; 71: 1220–6
Bird T, Nochlin D, Poorkaj P, et al. A clinical pathological comparison of three families with frontotemporal dementia and identical mutations in the tau gene (P301L). Brain 1999; 122 (Pt 4): 741–56
Bugiani O, Murrell JR, Giaccone G, et al. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol 1999; 58: 667–77
Geschwind DH, Robidoux J, Alarcon M, et al. Dementia and neurodevelopmental predisposition: cognitive dysfunction in presymptomatic subjects precedes dementia by decades in frontotemporal dementia. Ann Neurol 2001; 50: 741–6
Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 1999; 8: 711–5
Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 2001; 56: 1702–6
He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 2003; 81: 600–12
He Z, Ong CH, Halper J, et al. Progranulin is a mediator of the wound response. Nat Med 2003; 9: 225–9
Ahmed Z, Mackenzie IR, Hutton ML, et al. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation 2007; 4: 7
Suzuki M, Nishiahara M. Granulin precursor gene: a sex steroid-inducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab 2002; 75: 31–7
Van Damme P, Van Hoecke A, Lambrechts D, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol 2008; 181: 37–41
Kayasuga Y, Chiba S, Suzuki M, et al. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res 2007; 185: 110–8
Chen-Plotkin AS, Geser F, Plotkin JB, et al. Variations in the progranulin gene affect global gene expression in frontotemporal lobar degeneration. Hum Mol Genet 2008; 17: 1349–62
Le Ber I, van der Zee J, Hannequin D, et al. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 2007; 28: 846–55
Yu CE, Bird TD, Bekris LM, et al. The spectrum of mutations in progranulin. Arch Neurol 2010; 67(2): 161–70
Le Ber I, Camuzat A, Hannequin D, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 2008; 131: 732–46
Rademakers R, Baker M, Gass J, et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C→T (Arg493X) mutation: an international initiative. Lancet Neurol 2007; 6: 857–68
Snowden JS, Pickering-Brown SM, Mackenzie IR, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain 2006; 129: 3091–102
Whitwell JL, Jack Jr CR, Baker M, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch Neurol 2007; 64: 371–6
Rohrer JD, Warren JD, Omar R, et al. Parietal lobe deficits in frontotemporal lobar degeneration caused by a mutation in the progranulin gene. Arch Neurol 2008; 65: 506–13
Beck J, Rohrer JD, Campbell T, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain 2008; 131: 706–20
Zhang YJ, Xu YF, Dickey CA, et al. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci 2007; 27: 10530–4
Dormann D, Capell A, Carlson AM, et al. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem 2009; 110: 1082–94
Skibinski G, Parkinson NJ, Brown JM, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 2005; 37: 806–8
Gydesen S, Brown JM, Brun A, et al. Chromosome 3 linked frontotemporal dementia (FTD-3). Neurology 2002; 59: 1585–94
Parkinson N, Ince PG, Smith MO, et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 2006; 67: 1074–7
Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosincontaining protein. Nat Genet 2004; 36: 377–81
Kimonis VE, Mehta SG, Fulchiero EC, et al. Clinical studies in familial VCP myopathy associated with Paget disease of bone and frontotemporal dementia. Am J Med Genet A 2008; 146A: 745–57
Blair IP, Williams KL, Warraich ST, et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. Epub 2009 Dec 3
Morita M, Al-Chalabi A, Andersen PM, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 2006; 66: 839–44
Vance C, Al-Chalabi A, Ruddy D, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain 2006; 129: 868–76
Van Deerlin V, Martinez-Lage M, Hakonarson H, et al. Genome-wide association study of frontotemporal lobar degeneration with or without concomitant motor neuron disease and TDP-43 neuropathology [abstract]. 6th International Conference on Frontotemporal Dementias; Sep 2008 3–5; Rotterdam
Davies RR, Kipps CM, Mitchell J, et al. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol 2006; 63: 1627–31
Kipps CM, Nestor PJ, Dawson CE, et al. Measuring progression in frontotemporal dementia: implications for therapeutic interventions. Neurology 2008; 70: 2046–52
Woolley JD, Wilson MR, Hung E, et al. Frontotemporal dementia and mania. Am J Psychiatry 2007; 164: 1811–6
Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006; 59: 952–62
Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain 2007; 130: 2636–45
Knibb JA, Xuereb JH, Patterson K, et al. Clinical and pathological characterization of progressive aphasia. Ann Neurol 2006; 59: 156–65
Johnson J, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol 1999; 56: 1233–9
Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008; 63: 709–19
Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008; 64: 388–401
McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65: 1863–72
Boxer AL, Rankin KP, Miller BL, et al. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Arch Neurol 2003; 60: 949–56
Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 2001; 286: 2120–7
Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 2007; 130: 2616–35
Nestor P, Hodges J. Non-Alzheimer dementias. Semin Neurol 2000; 20: 439–46
Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2003; 2: 605–13
Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid 1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 2003; 289: 2094–103
Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology 2008; 70: 1827–35
Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–19
Rabinovici GD, Furst AJ, O’Neil JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2007; 68: 1205–12
Kim EJ, Rabinovici GD, Seeley WW, et al. Patterns of MRI atrophy in tau positive and ubiquitin positive frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2007; 78:1375–8
Whitwell JL, Josephs KA, Rossor MN, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol 2005; 62: 1402–8
Grossman M, Libon DJ, Forman MS, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch Neurol 2007; 64: 1601–9
Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 2006; 66: 17–22
Boxer AL, Boeve BF. Frontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies. Alzheimer Dis Assoc Disord 2007; 21: S79–87
Vossel KA, Miller BL. New approaches to the treatment of frontotemporal lobar degeneration. Curr Opin Neurol 2008; 21: 708–16
Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008; 131: 2957–68
Engelborghs S, Vloeberghs E, Maertens K, et al. Evidence for an association between the CSF HVA:5-HIAA ratio and aggressiveness in frontotemporal dementia but not in Alzheimer’s disease [letter]. J Neurol Neurosurg Psychiatry 2004; 75: 1080
Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol 2001; 101: 256–70
Sparks D, Markesbery WR. Altered serotonergic and cholinergic synaptic markers in Pick’s disease. Arch Neurol 1991; 48: 796–9
Procter AW, Qurne M, Francis PT. Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord 1999; 10 Suppl. 1: 80–4
Bowen DM, Procter AW, Mann DM, et al. Imbalance of a serotonergic system in frontotemporal dementia: implication for pharmacotherapy. Psychopharmacology (Berl) 2008; 196: 603–10
Franceschi M, Anchisi D, Pelati O, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol 2005; 57: 216–25
Swartz JR, Miller BL, Lesser IM, et al. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors [published erratum appears in J Clin Psychiatry 1997 Jun; 58 (6): 275]. J Clin Psychiatry 1997; 58: 212–6
Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord 2004; 17: 117–21
Moretti R, Torre P, Antonello RM, et al. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol 2003; 49: 13–9
Deakin JB, Rahman S, Nestor PJ, et al. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl) 2004; 172: 400–8
Lebert F, Stekke W, Hasenbroekx C, et al. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord 2004; 17: 355–9
Moretti R, Torre P, Antonello RM, et al. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Demen 2003; 18: 205–14
Curtis RC, Resch DS. Case of pick’s central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J Clin Psychopharmacol 2000; 20: 384–5
Fellgiebel A, Muller MJ, Hiemke C, et al. Clinical improvement in a case of frontotemporal dementia under aripiprazole treatment corresponds to partial recovery of disturbed frontal glucose metabolism. World J Biol Psychiatry 2007; 8: 123–6
Pijnenburg YA, Sampson EL, Harvey RJ, et al. Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. Int J Geriatr Psychiatry 2003; 18: 67–72
US FDA [online]. Available from URL: http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm053171.htm [Accessed 2010 Feb 17]
Hansen LA, Deteresa R, Tobias H, et al. Neocortical morphometry and cholinergic neurochemistry in Pick’s disease. Am JPathol 1988; 131: 507–18
Meier-Ruge W, Iwangoff P, Reichlmeier K. Neurochemical enzyme changes in Alzheimer’s and Pick’s disease. Arch Gerontol Geriatr 1984; 3: 161–5
Yates CM, Simpson J, Maloney AFJ, et al. Neurochemical observations in a case of Pick’s disease. Neurol Sci 1980; 48: 257–63
Moretti R, Torre P, Antonello RM, et al. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging 2004; 21: 931–7
Kertesz A, Morlog D, Light M, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord 2008; 25: 178–85
Mendez MF, Shapira JS, McMurtray A, et al. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry 2007; 15: 84–7
Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348: 1333–41
Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord 2007; 21: 164–6
Diehl-Schmid J, Forstl H, Perneczky R, et al. A 6-month, open-label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry 2008; 23: 754–9
Boxer AL, Lipton AM, Womack K, et al. An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord 2009 Jul–Sep; 23(3): 211–7
van Balken I, Litvan I. Current and future treatments in progressive supranuclear palsy. Curr Treat Options Neurol 2006; 8: 211–23
Litvan I, Grimes DA, Lang AE, et al. Clinical features differentiating patients with postmortem confirmed progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999; 246 Suppl. 2: I 1–5
Bensimon G, Lacomblez L, Meininger V. on behalf of the ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med 1994; 330: 585–91
Lomen-Hoerth C. Amyotrophic lateral sclerosis from bench to bedside. Semin Neurol 2008; 28: 205–11
Merrilees J. A model for management of behavioral symptoms in frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord 2007; 21: S64–9
Talerico KA, Evans LK. Responding to safety issues in frontotemporal dementias. Neurology 2001; 56: S52–5
Rademakers R, Cruts M, van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 2004; 24: 277–95
Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 2000; 25: 402–5
Lin WL, Lewis J, Yen SH, et al. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol 2003; 32: 1091–105
Wittmann CW, Wszolek MF, Shulman JM, et al. Tauopathy in drosophila: neurodegeneration without neurofibrillary tangles. Science 2001; 293: 711–4
Kraemer BC, Zhang B, Leverenz JB, et al. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci U S A 2003; 100: 9980–5
Trojanowski JQ, Duff K, Fillit H, et al. New directions for frontotemporal dementia drug discovery. Alzheimers Dement 2008; 4: 89–93
Rademakers R, Eriksen JL, Baker M, et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 2008; 17: 3631–42
Acknowledgements
The authors are supported by the National Institute on Aging grants K23-AG031861 (Gil D. Rabinovici), P01-AG1972403 (Bruce L. Miller), P50-AG023501 (Bruce L. Miller), Alzheimer’s Association grant NIRG-07-59422 (Gil D. Rabinovici) and the John Douglas French Alzheimer’s Association (Gil D. Rabinovici).
The authors have no conflicts of interest that are directly relevant to the content of this review. Dr Rabinovici has received personal compensation for serving on scientific advisory boards for General Electric Healthcare and Novartis Diagnostics. Dr Miller has received personal compensation for participating in the speaker’s bureau for Novartis Pharmaceuticals and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabinovici, G.D., Miller, B.L. Frontotemporal lobar degeneration. CNS Drugs 24, 375–398 (2010). https://doi.org/10.2165/11533100-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11533100-000000000-00000