Abstract
Background: The Thai healthcare setting has seen patients with cervical cancer experience an increasing burden of morbidity and mortality, a stagnation in the performance of cervical screening programmes and the introduction of a vaccine for the prevention of human papillomavirus (HPV) infection.
Objective: This study aims to identify the optimum mix of interventions that are cost effective, from societal and healthcare provider perspectives, for the prevention and control of cervical cancer.
Methods: A computer-based Markov model of the natural history of cervical cancer was used to simulate an age-stratified cohort of women in Thailand. The strategy comparators, including both control and prevention programmes, were (i) conventional cytology screening (Pap smears); (ii) screening by visual inspection with acetic acid (VIA); and (iii) HPV-16, -18 vaccination. Input parameters (e.g. age-specific incidence of HPV infection, progression and regression of the infection, test performance of screening methods and efficacy of vaccine) were synthesized from a systematic review and metaanalysis. Costs (year 2007 values) and outcomes were evaluated separately, and compared for each combination. The screening strategies were started from the age of 30–40 years and repeated at 5- and 10-year intervals. In addition, HPV vaccines were introduced at age 1560 years.
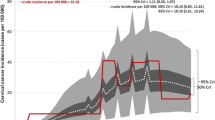
Results: All of the screening strategies showed certain benefits due to a decreased number of women developing cervical cancer versus no intervention. Moreover, the most cost-effective strategy from the societal perspective was the combination of VIA and sequential Pap smear (i.e. VIA every 5 years for women aged 3045 years, followed by Pap smear every 5 years for women aged 5060 years). This strategy was dominant, with a QALY gain of 0.01 and a total cost saving of Baht (Bt)800, compared with doing nothing. From the societal perspective, universal HPV vaccination for girls aged 15 years without screening resulted in a QALY gain of 0.06 at an additional cost of Bt8800, based on the cost of Bt15 000 for a full immunization schedule. The incremental cost-effectiveness ratio, comparing HPV vaccinations for girls aged 15 years with the current national policy of Pap smears for women aged 3560 years every 5 years, was approximately Bt181 000 per QALY gained. This figure was relatively high for the Thai setting.
Conclusions: The results suggest that controlling cervical cancer by increasing the numbers of women accepting the VIA and Pap smear screening as routine and by improving the performance of the existing screening programmes is the most cost-effective policy option in Thailand.









Similar content being viewed by others
References
Bundhamcharoen K, Teerawattananon Y, Vos T, et al. Burden of disease and injuries in Thailand, priority setting for policy. Nonthaburi: Bureau of Health Policy and Planning, Ministry of Public Health, 2002
Working group of Burden of Disease Project. Burden of disease and injuries in Thailand, 2004 (interim report). Nonthaburi: International Health Policy Program, Ministry of Public Health, 2007
Sriplung H, Wiangnon S, Sontipong S, et al. Cancer incidence trends in Thailand, 1989–2000. Asian Pac J Cancer Prev 2006 Apr-Jun; 7 (2): 239–44
Report of 2006 reproductive health survey. Bangkok: National Statistical Office, Ministry of Information and Communication Technology, 2006
IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: International Agency for Reseahttp://www.price.moc.go.th/price/cpi/index_new_e.asprch on Cancer, 2005
Schiffman M, Kjaer SK. Chapter 2: natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr 2003; 31: 14–9
Yothasamut J, Putchong C, Sirisamutr T, et al. Scaling up cervical cancer screening in the midst of human papillomavirus vaccination advocacy in Thailand. Health Serv Res 2010; 10 Suppl. 1: S5
Arbyn M, Sasieni P, Meijer CJ, et al. Chapter 9: clinical applications of HPV testing. A summary of meta-analyses. Vaccine 2006 Aug 21; 24 Suppl. 3: S78–89
The Committee of Gynecologic Oncology. Clinical practice guidelines for cervical cancer screening. Bangkok: The Royal Thai College of Obstetricians and Gynecologists, 2006
Gaffikin L, Blumenthal PD, Emerson M, et al. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet 2003 Mar 8; 361 (9360): 814–20
Limwattananon S. Current performance of the Cervical Cancer Prevention and Control Program in Thailand. Bangkok: International Health Policy Program, Health Intervention and Technology Assessment Program, Population and Reproductive Health Capacity Building Program, The World Bank, 2007 Aug 20
Eliav B, Heather LS. Prophylactic HPV vaccines: new interventions for cancer control. Vaccine 2008 Nov 18; 26 (49): 6244–57
US National Institutes of Health, National Cancer Institute. National Cancer Institute Fact Sheet. Human papillomavirus (HPV) vaccines [online]. Available from URL: http://www.cancer.gov/cancertopics/factsheet/Prevention/HPVvaccine [Accessed 2009 Nov 30]
Briggs AH, Sculpher MJ, Claxton K. Decision modelling for health economic evaluation. New York: Oxford University Press, 2006
Benedet J, Bender H, Jones HI, et al. FIGO staging classifications and clinical practice guidelines in the management of gynaecologic cancers. Int J Gynaecol Obstet 2000; 70 (2): 209–62
Permsuwan U, Guntawongwan K, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thai 2008; 91 Suppl. 2: S53–8
Myers ER, McCrory DC, Nanda K, et al. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol 2000 Jun 15; 151 (12): 1158–71
Sritipsukho P. The systematic review of the operating characteristics of screening tests including VIA, PAP smear, and HPV DNA testing. Bangkok: International Health Policy Program, Health Intervention and Technology Assessment Program, Population and Reproductive Health Capacity Building Program, The World Bank, 2007 Aug 20
Rambout L, Hopkins L, Hutton B, et al. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007 Aug 28; 177 (5): 469–79
Limwattananon S. The determination of the performance of the current programs for prevention and control of cervical cancer in Thailand. Bangkok: International Health Policy Program, Health Intervention and Technology Assessment Program, Population and Reproductive Health Capacity Building Program, The World Bank, 2007 Aug 20
Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Costeffectiveness of cervical-cancer screening in five developing countries. N Engl J Med 2005 Nov 17; 353 (20): 2158–68
Sukvirach S, Smith JS, Tunsakul S, et al. Population-based human papillomavirus prevalence in Lampang and Songkla, Thailand. J Infect Dis 2003 Apr 15; 187 (8): 1246–56
Khuhaprema T, Srivatanakul P, Sriplung H, et al. Cancer in Thailand. Vol. IV, 1998–2000. Bangkok: National Cancer Institute of Thailand, Ministry of Public Health, 2007
Tumour Registry Database: Cervical Cancer. 2000–4. Bangkok: The Thai Gynecologic Oncology Collaborative Group (TGOC), 2007
Bradburn MJ, Clark TG, Love SB, et al. Survival analysis part III: multivariate data analysis — choosing a model and assessing its adequacy and fit. Br J Cancer 2003; 89: 605–11
Report of 2003 health and welfare survey. Bangkok: National Statistical Office, Ministry of Information and Communication Technology, Thailand, 2003. Report No. S2-021
Chichareon S. Economic burden of life-time treatment cost, and quality of life among invasive cervical cancer patients treated at university hospitals and cancer centers in Thailand. In: Clinical Research Collaboration Network, The Thai Gynecologic Oncology Collaborative Group, editors. Nonthaburi, 2008 (Data on file; accessed 2008 Mar 30)
The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990; 16 (3): 199–208
Brooks R. EuroQol: the current state of play. Health Policy 1996; 37 (1): 53–72
Tongsiri S. The Thai population based preference scores for EQ5D health states. Thailand: Health Intervention and Technology Assessment Program (HITAP), 2009
Szende A, Oppe M, Devlin N, editors. EQ-5D value sets: inventory, comparative review and user guide. EuroQol Group Monographs, Vol. 2. New York (NY): Springer, 2006
Brouwer W, Rutten F, Koopmanschap M. Costing in economic evaluations. In: Drummond M, McGuire A, editors. Economic evaluation in health care: merging theory with practice. New York: Oxford University Press, 2001: 68–93
Ut-Ang K, Riewpaiboon A. Cost of logistics of vaccines in the expanded programme on immunization in Thailand. Bangkok: Mahidol University, 2009
Bureau of Trade and Economic Indices. Consumer price index database. Bangkok: Ministry of Commerce, 2008 April 2 [online]. Available from URL: [Accessed 2008 Jun 7]
International Monetary Fund. World economic and financial surveys, world economic outlook database: October 2007 edition [online]. Available from URL: http://www.imf.org/external/pubs/ft/weo/2007/02/weodata/index.aspx [Accessed 2007 Dec 10]
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000; 17 (5): 479–500
Daniels MJ, Zhao YD. Modelling the random effects covariance matrix in longitudinal data. Stat Med 2003 May 30; 22 (10): 1631–47
Zethraeus N, Johannesson M, Jönsson B, et al. Advantages of using the net-benefit approach for analysing uncertainty in economic evaluation studies. Pharmacoeconomics 2003; 21 (1): 39–48
Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005; 187: 106–8
Wibulpolprasert S. The need for guidelines and the use of economic evidence in decision-making in Thailand: lessons learnt from the development of the National List of Essential Drugs. J Med Assoc Thai 2008; 91 Suppl. 2: S1–3
Kulasingam SL, Benard S, Barnabas RV, et al. Adding a quadrivalent human papillomavirus vaccine to the UK cervical cancer screening programme: a cost-effectiveness analysis. Cost Eff Resour Alloc 2008 Feb 15; 6: 4
de Kok IM, van Ballegooijen M, Habbema JD. Costeffectiveness analysis of human papillomavirus vaccination in the Netherlands. J Natl Cancer Inst 2009; 101 (15): 1083–92
Sanders GD, Taira AV. Cost effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003; 9 (1): 37–48
Brisson M, Van de Velde N, De Wals P, et al. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007; 25 (29): 5399–408
Diaz M, Kim JJ, Albero G, et al. Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India. Br J Cancer 2008; 99 (2): 230–8
Goldie SJ, Kim JJ, Myers E. Chapter 19: cost-effectiveness of cervical cancer screening. Vaccine 2006 Aug 21; 24 Suppl. 3: S164–70
Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ 2004 May; 13 (5): 437–52
Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine 2006 Aug 21; 24 Suppl. 3: S11–25
Acknowledgements
This study was conducted with funding from the World Bank’s Population and Reproductive Health Capacity Building Program. The authors’ gratitude goes to experts and representatives from the TGOC and Clinical Research Collaboration Network (CRCN) who participated in the gathering of epidemiology and costing parameters.
The Health Intervention and Technology Assessment Program (HITAP) is supported by the Thai Health Promotion Foundation, the Health Systems Research Institute, the Bureau of Policy and Strategy and the Thai Health-Global Link Initiative Project. The findings and opinions in this report have not been endorsed by the above funding agencies and do not reflect the policy stance of these organizations.
N. Praditsitthikorn is currently doing her PhD at Mahidol University and this study is also a part of her PhD thesis. She also works as a researcher at HITAP.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Praditsitthikorn, N., Teerawattananon, Y., Tantivess, S. et al. Economic Evaluation of Policy Options for Prevention and Control of Cervical Cancer in Thailand. Pharmacoeconomics 29, 781–806 (2011). https://doi.org/10.2165/11586560-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11586560-000000000-00000