Abstract
Background: Lacosamide, a new antiepileptic drug (AED) with a different pharmacological action that enhances sodium channel slow inactivation, is approved for the adjunctive treatment of partial-onset seizures in adults. Previous analyses of pooled phase II/III trials have demonstrated that lacosamide provides additional efficacy when added to a broad range of AEDs.
Objective: To further evaluate the efficacy and safety of lacosamide by grouping patients based upon the sodium channel-blocking properties of their concomitant AEDs.
Study Design: Post hoc exploratory analyses were performed on pooled data in which patients were grouped based upon inclusion or non-inclusion of at least one ‘traditional’ sodium channel-blocking AED (defined as carbamazepine, lamotrigine, oxcarbazepine and phenytoin derivatives) as part of their concomitant AED regimen.
Setting: Data pooled from previously conducted phase II/III clinical trials of lacosamide.
Patients: Adult patients with partial-onset seizures with or without secondary generalization (N = 1308).
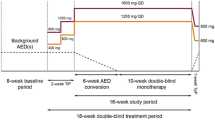
Intervention: Four- to six-week Titration Phase followed by 12-week maintenance treatment with adjunctive lacosamide (Vimpat®) [200, 400 or 600 mg/day] or placebo.
Main Outcome Measure: Efficacy variables included change in seizure frequency per 28 days and the proportion of patients experiencing a ≥50% reduction in seizure frequency (50% responder rate) from Baseline to the Maintenance Phase. The proportion of patients experiencing a ≥75% reduction in seizure frequency from Baseline to the Maintenance Phase (75% responder rate) was also assessed. Safety parameters assessed were treatment-emergent adverse events (TEAEs) and discontinuation due to TEAEs. Additional safety assessments were changes in ECG and laboratory parameters as well as vital signs (including bodyweight).
Results: Of 1308 patients in the pooled phase II/III population, the majority (82%) were using at least one ‘traditional’ sodium channel-blocking concomitant AED. In this subgroup of patients, adjunctive lacosamide showed significant reductions in seizure frequency (p<0.01, all dosages) and significantly greater 50% and 75% responder rates (p < 0.01 for 400 mg/day; p < 0.01 [50% responder rate] and p<0.05 [75% responder rate] for 600 mg/day) compared with placebo; these effects were similar to the results seen in the pooled phase II/III population. TEAEs and discontinuations due to TEAEs in this subgroup were dose related and similar to the pooled phase II/III population. In the remaining subgroup of patients, i.e. those not taking ‘traditional’ sodium channel-blocking AEDs as part of their concomitant AED regimen (n = 231; 18%), a pronounced, dose-related seizure reduction was observed with lacosamide (p<0.01, 400 and 600mg/day for median percent seizure reduction and 50% or 75% responder rates). Also in this group, incidences of TEAEs were low, and discontinuations due to TEAEs did not appear to increase with dose. Analyses of ECG, laboratory and vital signs (including bodyweight) assessments did not identify abnormalities in either subgroup that were outside of the known safety profile of lacosamide observed in the pooled phase II/III population.
Conclusion: In this post hoc exploratory analysis, adjunctive lacosamide demonstrated significant seizure reduction over placebo regardless of the inclusion of ‘traditional’ sodium channel blockers in the concomitant AED regimen. Future prospective studies evaluating single AED combinations (e.g. lacosamide plus one other drug) are needed to better evaluate the potential for additive or synergistic effects of lacosamide in combination with AEDs not considered ‘traditional’ sodium channel blockers.









Similar content being viewed by others
References
Kwan P, Brodie MJ. Combination therapy in epilepsy: when and what to use. Drugs 2006; 66(14): 1817–29
Deckers CL, Czuczwar SJ, Hekster YA, et al. Selection of antiepileptic drug polytherapy based on mechanisms of action: the evidence reviewed. Epilepsia 2000 Nov; 41(11): 1364–74
St Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol 2009 Jun; 7(2): 96–105
Fillit HM, Doody RS, Binaso K, et al. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother 2006; 4 Suppl. A: S9–24; quiz S5-S8
Krymchantowski AV, Bigal ME. Polytherapy in the preventive and acute treatment of migraine: fundamentals for changing the approach. Expert Rev Neurother 2006 Mar; 6(3): 283–9
McCrindle BW, Urbina EM, Dennison BA, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation 2007 Apr 10; 115(14): 1948–67
French JA, Faught E. Rational polytherapy. Epilepsia 2009 Sep; 50 Suppl. 8: 63–8
Jonker DM, Voskuyl RA, Danhof M. Synergistic combinations of anticonvulsant agents: what is the evidence from animal experiments? Epilepsia 2007 Mar; 48(3): 412–34
Brodie MJ, Mumford JP. Double-blind substitution of vigabatrin and valproate in carbamazepine-resistant partial epilepsy: 012 Study group. Epilepsy Res 1999 Apr; 34(2–3): 199–205
Stafstrom CE. Mechanisms of action of antiepileptic drugs: the search for synergy. Curr Opin Neurol 2010 Apr; 23(2): 157–63
Brodie M J, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res 1997 Mar; 26(3): 423–32
VIMPAT® (lacosamide): US prescribing information. Smyrna (GA): UCB, Inc., 2008
VIMPAT® (lacosamide): summary of product characteristics. Belgium: UCB Pharma, S.A., 2008
Beyreuther BK, Freitag J, Heers C, et al. Lacosamide: a review of preclinical properties. CNS Drug Rev 2007; 13(1): 21–42
Ben-Menachem E, Biton V, Jatuzis D, et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia 2007 Jul; 48(7): 1308–17
Chung S, Sperling MR, Biton V, et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia 2010 Jun; 51(6): 958–67
Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia 2009 Mar; 50(3): 443–53
Rosenfeld WE, Rudd D, Hebert D, et al. Lacosamide efficacy is independent of concomitant AED(s) treatment. 61st American Academy of Neurology Annual Meeting; 2009 Apr 25–May 2; Seattle (WA)
Brodie MJ, Schachter S, Kwan P. Epilepsy: fast facts. 3rd ed. Oxford: Health Press, 2005
Acknowledgements
Individual trials as well as pooled analyses were supported by SCHWARZ Biosciences, Inc. and SCHWARZ Biosciences GmbH, members of the UCB group. John-Kenneth Sake, MD, and Marc De Backer, MD, are employed by UCB Pharma SA, a member of the UCB group. Pamela Doty, PhD, David Hebert, PhD, and Jouko Isojarvi, MD, PhD, are employees of SCHWARZ BIOSCIENCES, a member of the UCB group. James Zackheim, PhD, is employed by UCB, Inc., a member of the UCB group. Kendra Davies, PharmD, BCPP, and Andrea Eggert-Formella, PharmD, BCPP, are former employees of UCB, Inc. The authors gratefully acknowledge the following for their expert commentary: Martin Brodie, Jacqueline French, Barry Gidal, Tony Marson, Emilio Perucca and Phillipe Ryvlin. Full medical writing assistance was supported by UCB and was provided by Jennifer Hepker, PhD, at Prescott Medical Communications Group (Chicago, IL, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sake, JK., Hebert, D., Isojärvi, J. et al. A Pooled Analysis of Lacosamide Clinical Trial Data Grouped by Mechanism of Action of Concomitant Antiepileptic Drugs. CNS Drugs 24, 1055–1068 (2010). https://doi.org/10.2165/11587550-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11587550-000000000-00000