- 1 Department of Microbiology and Immunology, College of Medicine, University of Louisville, Louisville, KY, USA
- 2 Department of Biological Sciences, University of Louisville, Louisville, KY, USA
- 3 Department of Microbiology, Al-Quds University Medical School, Abu Dies, Jerusalem, Palestinian territory
- 4 Department of Microbiology and Molecular Genetics, The Hebrew University Medical School, Jerusalem, Israel
The Dot/Icm-translocated Ankyrin B (AnkB) F-box effector of Legionella pneumophila is essential for intra-vacuolar proliferation and functions as a platform for the docking of polyubiquitinated proteins to the Legionella-containing vacuole (LCV) within macrophages and ameba. Here we show that ectopically expressed AnkB in Dictyostelium discoideum is targeted to the plasma membrane where it recruits polyubiquitinated proteins and it trans-rescues the intracellular growth defect of the ankB null mutant, which has never been demonstrated for any effector in ameba. Using co-immunoprecipitation and bimolecular fluorescence complementation we show specific interaction of Skp1 of D. discoideum with the F-box domain of AnkB, which has never been demonstrated in ameba. We show that anchoring of AnkB to the cytosolic face of the LCV membrane in D. discoideum is mediated by the host farnesylation of the C-terminal eukaryotic CaaX motif of AnkB and is independent of the F-box and the two ANK domains, which has never been demonstrated in ameba. Importantly, the three host farnesylation enzymes farnesyl transferase, RCE-1, and isoprenyl cysteine carboxyl methyl transferase of D. discoideum are recruited to the LCV in a Dot/Icm-dependent manner, which has never been demonstrated in ameba. We conclude that the polyubiquitination and farnesylation enzymatic machineries of D. discoideum are recruited to the LCV in a Dot/Icm-dependent manner and the AnkB effector exploits the two evolutionarily conserved eukaryotic machineries to proliferate within ameba, similar to mammalian cells. We propose that L. pneumophila has acquired ankB through inter-kingdom horizontal gene transfer from primitive eukaryotes, which facilitated proliferation of L. pneumophila within human cells and the emergence of Legionnaires’ disease.
Introduction
Legionella pneumophila is a facultative intracellular Gram-negative bacterium that is ubiquitous in aquatic environments (Fields, 1996; Harb et al., 2000; Bitar et al., 2004; Molmeret et al., 2005). L. pneumophila invades and replicates within fresh water amebae and ciliated protozoa. The co-evolution and bacterial adaptation to protozoan hosts is thought to be a factor for the ability of L. pneumophila to proliferate within human cells and cause disease (Harb et al., 2000; Swanson and Hammer, 2000; Molmeret et al., 2005). The transmission of L. pneumophila to humans takes place by inhalation of L. pneumophila-contaminated aerosols. L. pneumophila reaches the alveoli, where it infects and replicates within alveolar cells leading to an atypical pneumonia known as Legionnaires’ disease (Kaufmann et al., 1981). Remarkably, the life cycle of L. pneumophila within amebae and macrophages is similar (Fields et al., 2002). Within both host cells, the Legionella-containing vacuole (LCV) evades targeting to and degradation by the endosomal–lysosomal pathway and is remodeled by the endoplasmic reticulum (ER). During late stages of intracellular proliferation within macrophages and ameba, L. pneumophila disrupts the phagosomal membrane and escapes into the host cell cytosol where various virulence traits are triggered to enable egress of the bacteria to the extracellular environment (Molmeret et al., 2004, 2010; Al-Khodor et al., 2010).
Efficient formation of a replication vacuole and successful intracellular growth of L. pneumophila requires the Dot/Icm type IV secretion system (Purcell and Shuman, 1998; Vogel et al., 1998). It is estimated that >200 effectors are translocated into the host cell by the Dot/Icm secretion system, but most of the studied effectors are dispensable for intracellular proliferation (Isberg et al., 2009). The Dot/Icm-translocated AnkB effector is one of very few exceptions, since it plays a major role in intracellular proliferation within macrophages and protozoa and is essential for intrapulmonary proliferation of L. pneumophila in the mouse model (Al-Khodor et al., 2008, 2010; Habyarimana et al., 2008; Price et al., 2009). The majority of the structure of AnkB is composed of eukaryotic domains and motifs that include an F-box domain, two Ankyrin repeats and a C-terminal CaaX farnesylation motif (Al-Khodor et al., 2008, 2010; Habyarimana et al., 2008; Price et al., 2009).
Legionella pneumophila is one of many intracellular bacterial pathogens that exploit the host polyubiquitination machinery (Dorer et al., 2006; Price et al., 2009, 2010a,b; Al-Khodor et al., 2010). Ubiquitination is a highly conserved eukaryotic post-translational process that covalently links ubiquitin monomers to target the protein to proteasomal degradation or to modulate its function (Kerscher et al., 2006). We have shown that AnkB mimics the action of host cell F-box proteins by functioning as a platform for the docking of polyubiquitinated proteins to the LCV within evolutionarily distant hosts; macrophages and ameba (Price et al., 2009, 2010b; Al-Khodor et al., 2010). Moreover, the F-box domain of AnkB interacts with mammalian Skp1; a component of the SCF1 (Skp1, Cullin1, F-box) ubiquitin ligase complex (Zheng et al., 2002). However; it is not known whether AnkB interacts with Skp1 of ameba.
In addition to exploitation of the host cell polyubiquitination machinery by AnkB, L. pneumophila also exploits the host farnesylation machinery via the C-terminal CaaX motif of AnkB to anchor the F-box effector into the cytosolic face of the LCV membrane (Price et al., 2010b). Farnesylation is a post-translational modification of eukaryotic proteins, which involves farnesyl transferase (FTase)-mediated addition of a 15 carbon lipid moiety at the conserved cysteine residue of the CaaX motif (Wright and Philips, 2006). After farnesylation, the “aaX” tri-peptide is cleaved by an endoprotease (RCE1 protease; Boyartchuk et al., 1997) followed by carboxyl methylation by isoprenyl cysteine carboxyl methyl transferase (IcmT; Dai et al., 1998; Bergo et al., 2000). This post-translational modification process increases protein hydrophobicity to enable anchoring of a hydrophilic protein to the lipid bi-layer of membranes. It is not known whether farnesylation of AnkB occurs locally at the LCV within Dictyostelium discoideum through selective recruitment of the farnesylation enzymatic machinery or that it occurs at other cellular sites and is trafficked back to the LCV. It is also not known whether, in addition to the CaaX farnesylation motif, any of the three eukaryotic domains of AnkB are involved in specific targeting of AnkB to the LCV membrane within D. discoideum.
We show that anchoring of AnkB to the cytosolic face of the LCV membrane in D. discoideum is mediated by the ameba farnesylation machinery, and is independent of the three eukaryotic domains of AnkB (F-box and the two ANK domains). Importantly, the three farnesylation enzymes FTase, RCE-1, and IcmT of D. discoideum are recruited to the LCV in a Dot/Icm-dependent manner. We conclude that the farnesylation and polyubiquitination enzymatic machineries of D. discoideum are recruited to the LCV in a Dot/Icm-dependent manner and the AnkB effector exploits the two evolutionarily conserved eukaryotic machineries to proliferate within ameba and human cells.
Materials and Methods
Bacterial Strains and Cell Cultures
Legionella pneumophila serogroup I parental strain AA100/130b (ATCC BAA-74) and the isogenic mutants; dotA, ankB, in addition to complemented ankB mutants were described previously (Al-Khodor et al., 2008; Price et al., 2010a). They were grown for 72 h on buffered charcoal–yeast extract (BCYE) plates at 37°C with 5% of CO2. The plates used for the cultivation of dotA and ank mutant strains were supplemented with kanamycin at a concentration of 50 μg/ml, and when required, chloramphenicol at concentration of 5 μg/ml. Escherichia coli strain DH5α was used for cloning purposes.
Ameba Culture
Axenic A. polyphaga was cultured as adherent cells in PYG medium as previously described. The D. discoideum wild type strain AX2 was grown axenically at 24°C in HL5 medium supplemented with 0.6% penicillin–streptomycin and G418 20 μg/ml as needed at 24°C as we described previously (Clarke et al., 1980; Price et al., 2010b).
Intracellular Growth Kinetics
The infection of D. discoideum and A. polyphaga were performed as described previously (Solomon et al., 2000; Price et al., 2009, 2010b). Briefly, the exponentially growing A. polyphaga or D. discoideum were infected for 1 h with bacterial strains at a multiplicity of infection (MOI) of 10 and incubated at 24°C (D. discoideum) or 37°C (A. polyphaga). After 60 min of the infection, 50 μg/ml gentamicin was added to the medium for 1 h to kill extracellular bacteria. At the time point indicated, the infected cells were washed two times with PBS (A. polyphaga) or SorC buffer (D. discoideum), and were lysed with 0.04% Triton X-100. A dilution series of the cell lysates was plated on CYE medium for 3 days. The number of bacteria was expressed as the number of CFU/ml. At least three independent experiments, in triplicate, were performed.
Confocal Laser Scanning Microscopy
Analyses of infected cells by confocal microscopy were performed as described previously for both hosts (Habyarimana et al., 2008; Price et al., 2009, 2010b). Briefly, at the time point indicated, the infected cells were washed three times with cold SorC buffer (D. discoideum) or PBS (A. polyphaga) and fixed with 4% paraformaldehyde in PBS for 30 min. The fixed cells were washed and were permeabilized (cold methanol 30 s) and blocked for 60 min. The 3XFLAG-tagged proteins were labeled with polyclonal rabbit anti-AnkB (1/200 dilution) antiserum, followed by Alexa-Fluor 488-conjugated donkey secondary anti-rabbit IgG antibody (Invitrogen, Carlsbad, CA, USA). Bacteria were labeled with monoclonal anti-L. pneumophila antibodies and Alexa-Fluor 647-conjugated donkey anti-mouse antibody. For ectopic expression of FLAG-ankB in D. discoideum; FLAG-ankB was labeled with polyclonal rabbit anti-AnkB (1/200 dilution) antiserum. To label the polyubiquitinated proteins, anti-polyubiquitin FK1 mouse monoclonal antibodies were used (BIOMOL International/Affiniti, Exeter, UK), followed by appropriate Alexa-Fluor conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA). Polyclonal rabbit anti-(FT-α or RCE-1) or polyclonal goat anti-IcmT antisera were used at 1:50 dilutions (Santa Cruz). The cells were examined by Olympus Fv1000 laser scanning confocal microscope as we described previously. On average, 8–15, 0.2 μm serial Z sections of each image were captured and stored for further analyses, using Adobe Photoshop CS3.
Isolation of LCVs
Phagosomes were isolated from post-nuclear supernatants (PNS) of infected D. discoideum as we described previously (Berger and Isberg, 1993; Price et al., 2009, 2010b). Post-exponentially grown L. pneumophila were introduced onto monolayers at MOI of 10 by 10 min centrifugation at 300×g, the infection was allowed to proceed for 1 h. After removing the extracellular bacteria by washing the cells three times with 10 ml of cold SorC, infected cells were scraped from the dish using 10 ml of cold SorC. The cells were pelleted by centrifugation (5 min, 1000 rpm, 4ºC), and re-suspended in 2 ml of homogenization buffer (20 mM Hepes/KOH pH = 7.2, 250 mM sucrose, 5 mM EGTA) containing protease inhibitors (Protease Inhibitor Cocktail, Sigma) and lysed in a Dounce homogenizer. The homogenate was transferred to microfuge tubes to separate LCVs from unbroken cells and nuclei (3 min, 1500 rpm, 4ºC). The PNS containing the LCVs was spun for 5 min at 4ºC onto poly l-lysine coated coverslips, and immobilized by 4% paraformaldehyde for 60 min. The LCVs were labeled with polyclonal rabbit anti-AnkB (1/200 dilution) antiserum and L. pneumophila was labeled with DAPI stain followed by Alexa-Fluor tagged anti-rabbit IgG secondary antibodies (Invitrogen, Carlsbad, CA, USA).
Chemical Inhibition of Farnesylation
The inhibitor FTI-277 was re-suspended in DMSO + 0.4 mM DTT and used immediately for experimental assays, as we described previously. D. discoideum were treated with 5.0 μM FTI-277 1 h before the infection. L. pneumophila was used to infect D. discoideum at MOI of 10 for 1 h followed by treatment with 50 μg/ml gentamicin for 1 h to kill extracellular bacteria. FTI-277 was maintained in the growth media throughout the experiment. After 2 h, semi-purified LCVs were purified and were fixed. Samples were labeled with polyclonal goat anti-Legionella and rabbit anti-AnkB antisera. Alexa-Fluor 488-tagged antibodies against rabbit IgG and Alexa-Fluor 555-conjugated donkey anti-goat IgG antibodies were used as secondary antibodies (Invitrogen, Carlsbad, CA, USA).
In vivo Co-Immunoprecipitation
Dictyostelium discoideum were infected with L. pneumophila strains for 2 h using MOI 50. Semi-purification of LCVs was performed as mentioned above. The supernatants that contain the semi-purified LCVs were incubated overnight at 4ºC with polyclonal rabbit anti-AnkB antibodies. One hundred microliter of immobilized protein G (Pierce, Rockford, IL, USA) were added to the reaction and incubated for 4 h at 4ºC. After removing the unbound proteins by washing the beads five times with cold PBS, samples were heated at 96°C for 5 min in sample buffer and subjected to 10.4–15% gradient SDS-PAGE gel electrophoresis. For AnkB-Skp1 interaction, samples were subjected to immunoblot analysis using an polyclonal rabbit anti-AnkB antibodies (1/60000 dilution; Price et al., 2010b) followed by anti-Skp1 (1/200 dilution) antibodies. To test if AnkB is being modified by farnesylation; samples were immunoblotted with anti-AnkB antibodies followed by anti-farnesylation antibodies (1/200 dilution). Immunoblots were visualized using SuperSignal West Femto substrate (Thermo Scientific).
BiFC and Transfection of Dictyostelium
To generate D. discoideum fusion constructs; total RNA was extracted from D. discoideum using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) as recommended by the manufacturer. Total RNA was treated with DNase I (Ambion, Austin, TX, USA) at 37°C for 30 min. Total RNA was used for cDNA synthesis with Superscript III Plus RNase H reverse transcriptase (RT; Invitrogen, Carlsbad, CA, USA) and random primers. Primers listed in Table 1 were used to amplify Skp1 from the generated cDNA. The resulting PCR products were cloned in pDM314 (Veltman et al., 2009) to generate GST-Skp1 fusion or pDXA-CFP/YFP to generate Skp1-YC (Knetsch et al., 2002) using the standard procedure.
To create NY-ankB and NY-ankB ΔF-box in pDXA-CFP/YFP; the ankB gene was amplified from the genome of L. pneumophila AA100/130b strain. The pBCSK+ vectors that harbors ankB-ΔF-box mutant alleles was used as templates to generate ankB-ΔF-box fusion as described previously (Price et al., 2009). Primers were used are listed in Table 1. The PCR product was treated with restriction enzymes that are mentioned in the table and was sub-cloned. The ligation products were transformed into E. coli DH5α. D. discoideum were transfected by electroporation following standard protocols (Pang et al., 1999). Cells were harvested at log phase and washed two times in cold H50 buffer and re-suspended in H50 at a concentration of 2 × 107 cells/ml. One hundred microliters of cells was then added to a cold 1-mm electroporation cuvette containing 4 μg of plasmid DNA. Cells and DNA were mixed and then incubated on ice for 5 min. Two consecutive pulses of 0.85 kV with a capacitance of 25 mF were applied to the cuvette with a 5-s recovery between pulses. After 5 min of incubation on ice, the cells from each transformation were plated onto a 100-mm culture dish containing 10 ml of HL5 and were allowed to recover for 24 h. Then, the medium was replaced by HL5 containing G418 20 μg/ml.
Statistical Analysis
All experiments were performed at least three times and the data shown are representatives of one experiment. To analyze for statistically significant differences between different sets of data, the two-tail Student’s t-test was used and the p-value was obtained.
Result
Ectopically Expressed AnkB in D. discoideum mediates docking of Polyubiquitinated Proteins to the Plasma Membrane and Trans-Rescues the AnkB Null Mutant
We have previously shown that AnkB functions as platforms for the docking of polyubiquitinated proteins to the LCVs within D. discoideum and macrophages (Price et al., 2009, 2010a; Al-Khodor et al., 2010). We examined whether an ectopically expressed FLAG-tagged AnkB in D. discoideum exhibited functional activity in recruitment of polyubiquitinated proteins. Co-localization of FLAG-AnkB with polyubiquitinated proteins was observed at the plasma membrane of FLAG-AnkB-transfected D. discoideum, where AnkB was exclusively localized (Figure 1). To determine the role of the F-box domain and its two conserved LP residues in the biological function of ectopically expressed AnkB, we transfected D. discoideum with the FLAG-AnkB-9L10P/AA or the FLAG-AnkB-ΔF-box constructs. The data showed that the F-box domain of AnkB was indispensable for the biological function of the effector, since ectopically expressed FLAG-AnkB-9L10P/AA and FLAG-AnkB-ΔF-box proteins failed to function as platforms for the docking of polyubiquitinated proteins despite their localization to the host plasma membrane (Figure 1). The C-terminal CaaX farnesylation motif of AnkB was indispensable for targeting AnkB to the plasma membrane, since ectopically expressed FLAG-AnkB169C/A failed to be targeted to the plasma membrane, which resulted in loss of biological function in recruiting polyubiquitinated proteins to the plasma membrane (Figure 1). Interestingly, FLAG-AnkBΔA1,2 ectopically expressed in D. discoideum was localized to the plasma membrane and also distributed throughout the cytosol, but it had no biological function as it failed to recruit polyubiquitinated proteins. We conclude that ectopically expressed AnkB in D. discoideum is biologically functional as platforms for the docking of polyubiquitinated proteins to the plasma membrane. The farnesylation motif and the ANK domains are required for targeting AnkB to the plasma membrane of D. discoideum. However, the F-box domain is not involved in localization of ectopically expressed AnkB to the plasma membrane of D. discoideum.
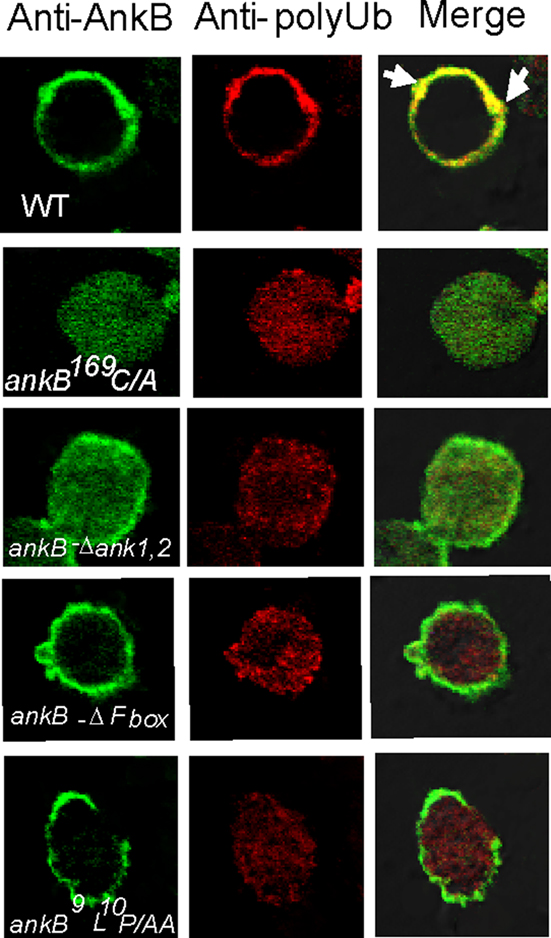
Figure 1. Biological function of AnkB as platforms for the recruitment of polyubiquitinated proteins to the plasma membrane of AnkB-transfected D. discoideum. D. discoideum was transfected with the FLAG-AnkB, or FLAG-AnkB169C/A, FLAG-ΔAnkB1,2, FLAG-ΔF-box, or AnkB9L10P/AA. Localization of FLAG-AnkB fusion proteins was examined by confocal laser scanning microscopy. Cells were labeled with anti-AnkB antibodies (green) and anti-polyubiquitin antibodies (red). The data are representatives of three independent experiments.
To examine whether the ectopically expressed AnkB would restore intracellular proliferation to the ankB mutant, D. discoideum cells transfected with FLAG-AnkB were infected with either the wild type strain AA100 or the ankB null mutant for 1 h, incubated for a total of 2 and 12 h and analyzed by microscopy for formation of replicative vacuoles, as we described previously (Price et al., 2009). The data revealed that there was no detectable proliferation of the ankB mutant in untransfected D. discoideum. However, the ankB mutant replicated similar to the wild type strain by 12 h post-infection of transfected D. discoideum (Figure 2). The dotA translocation-defective mutant was not trans-rescued by ectopically expressed FLAG-AnkB (data not shown). Interestingly, the ectopically expressed FLAG-AnkB protein was detected only at the plasma membrane but not on the LCV. We conclude that ectopically expressed AnkB in D. discoideum exhibits its biological function by acting as a platform for the docking of polyubiquitinated proteins at the plasma membrane and that is sufficient to trans-rescue the intracellular growth defect of the ankB mutant within the LCV. This is the first demonstration of a trans-rescue of a mutant of L. pneumophila defective in intracellular proliferation by ectopic expression of the mutated gene in ameba.
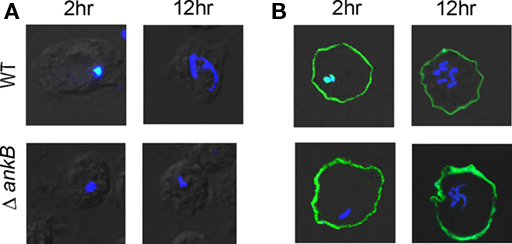
Figure 2. Trans-rescue of the ankB null mutant for intra-ameba growth defect in AnkB-transfected D. discoideum. Representative confocal microscopy images of D. discoideum to determine the formation of replicative LCVs. (A) Untransfected and (B) Transfected D. discoideum with FLAG-AnkB were infected with either the WT strain or the ΔankB mutant for 1 h and examined at 2 and 12 h post-infection. Cells were labeled with anti-Lpn antibodies (blue) and anti-AnkB antibodies (green). Rescue was determined by the observations of replicative vacuoles for the ankB mutant. The data are representatives of three independent experiments.
Interaction of Skp1 of D. discoideum with AnkB in vivo
We have recently shown that AnkB interacts with the mammalian Skp1, but whether AnkB interacts with Skp1 of ameba is not known. Therefore, a bimolecular fluorescence complementation (BiFc) approach was used to determine whether AnkB interacts with Skp1 of D. discoideum in vivo. In the BiFc approach, the yellow fluorescence protein (YFP) is expressed as N-terminal (YN) and C-terminal (YC) non-fluorescent fragments. Restoration of YFP fluorescence occurs when the two fragments are brought into proximity by an interaction between two proteins that have been fused to the YN and YC fragments, respectively (Hu et al., 2002). Either ankB-YN or ankB-ΔF-box–YN fusion proteins were co-expressed with YC–skp1 in D. discoideum. As a negative control; untransfected cells were used to rule out any auto-fluorescence. The results showed that a fluorescent protein was detected in cells transformed with AnkB–YN and YC–Skp1. Importantly, the F-box domain of AnkB was essential for Skp1-AnkB interaction, since the AnkB-ΔF-box–YN fusion did not interact with YC–Skp1, which confirmed the specificity of the interaction (Figure 3A).
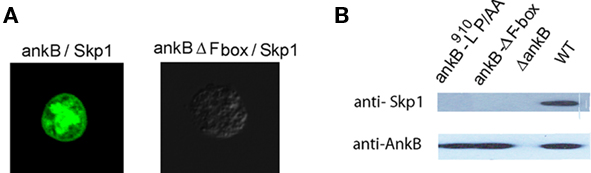
Figure 3. In vivo interaction of AnkB with Skp1 of D. discoideum. (A) Representative confocal images of co-transfected D. discoideum with constructs expressing fusions of AnkB-YN and Skp1-YC or AnkB-ΔF-box-YN and Skp1-YC. The data are representatives of three independent experiments. (B) D. discoideum were infected with L. pneumophila strains for 2 h. Skp1 was immunoprecipitated from semi-purified LCVs using anti-AnkB antibodies and then analyzed by immunoblotting with anti-AnkB antibodies followed by anti-Skp1 antibodies. The experiments were performed twice and representative examples are shown.
To confirm the BiFC results, the LCVs were semi-purified at 2 h post-infection of D. discoideum and were processed for Co-IP using anti-AnkB antibodies and analyzed by western blots probed with anti-Skp1 and anti-AnkB antibodies. The results showed that endogenous Skp1 of D. discoideum interacted with AnkB. In contrast, the AnkB-ΔF-box or AnkB-9L10P/AA variants failed to interact with Skp1 (Figure 3B). Taken together, we conclude that the F-box domain of AnkB interacts specifically with Skp1 of D. discoideum in vivo.
Post-Translational Modification of AnkB by the Farnesylation Machinery of D. discoideum and its Role in Anchoring AnkB to the LCV Membrane
We have previously shown that substitution of the cysteine residue in the CaaX motif with alanine (AnkB169C/A) abolishes anchoring of AnkB to the LCV membrane (Price et al., 2010b). It is not known whether the LCV-anchored AnkB was farnesylated by the host farnesylation machinery. To test if AnkB anchored to the LCV membrane was modified by the farnesylation machinery of D. discoideum, we infected D. discoideum with the wild type strain, the ankB null mutant, the ankB169C/A mutant or the translocation-defective dotA mutant as a negative control. Co-immunoprecipitation of semi-purified LCVs was performed using anti-AnkB antibodies, followed by western blots probed with anti-AnkB followed by anti-farnesyl antibodies. The data showed that AnkB but not the AnkB169C/A variant was detected by anti-farnesyl antibodies (Figure 4). As expected, AnkB expressed by the translocation-defective dotA mutant was not farnesylated.
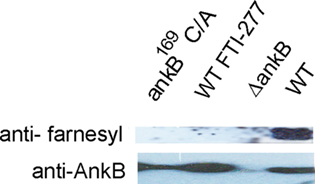
Figure 4. Ankyrin B is modified by the host cell farnesylation machinery. D. discoideum were infected with the L. pneumophila strains. The infection was performed for 1 h and the cells were examined at 2 h post-infection. The AnkB proteins were immunoprecipitated from semi-purified LCVs using anti-AnkB antibodies and then analyzed by immunoblotting with anti-AnkB and by anti-farnesyl antibodies. The data are representatives of independent experiments.
To confirm farnesylation of AnkB on the LCV within D. discoideum, immunoprecipitation was performed on LCVs harvested from D. discoideum that was pre-treated with the FTase inhibitor FTI-277 (Lerner et al., 1995). The data showed that inhibition of FTase blocked recognition of AnkB by the anti-farnesyl antibodies, similar to the AnkB169C/A variant in non-treated cells (Figure 4). We conclude that AnkB anchored to the LCV membrane is farnesylated by D. discoideum.
Inhibition of the farnesylation machinery has been shown to block intracellular proliferation (Price et al., 2010b). We examined whether inhibition of the FTase of D. discoideum by FTI-277 would prevent anchoring AnkB to the cytosolic face of LCV membrane. Therefore, D. discoideum was infected with the wild type strain AA100, the ankB null mutant or the ankB 169C/A mutant. The LCVs were isolated from untreated or FTI-277-treated D. discoideum to determine whether AnkB was localized to the cytosolic face of the LCV membrane. The inhibitor had no effect on viability of the cells (data not shown). AnkB was labeled with anti-AnkB antibodies prior to or after permeabilization of membranes. In untreated cells, permeabilized and non-permeabilized LCVs containing the ankB mutant failed to bind the anti-AnkB antibodies. Prior to permeabilization of membranes, the LCVs harboring the WT strain bound the anti-AnkB antibodies (Figure 5). In untreated cells, the LCVs harboring the ankB169C/A mutant did not bind the anti-AnkB antibodies prior to permeabilization but did bind after permeabilization. Importantly, in LCVs that harbored the WT strain isolated from FTI-277-treated D. discoideum, AnkB was not anchored to the cytosolic face of the LCV membrane, similar to the ankB169C/A mutant in untreated cells. We conclude that farnesylation of AnkB by D. discoideum is essential for anchoring AnkB to the cytosolic face of the LCV membrane.
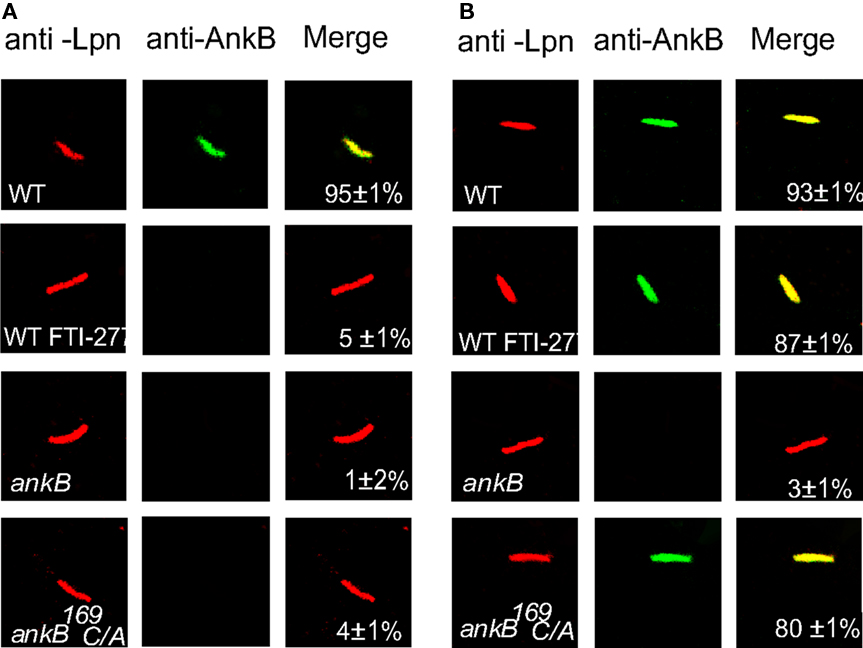
Figure 5. Farnesylation by D. discoideum anchors AnkB to the LCV membrane. The infection was performed for 1 h and the cells were lysed at 2 h post-infection. The LCVs were isolated from untreated or FTI-277-treated D. discoideum. The LCVs were labeled with (A) anti-AnkB antibodies prior to permeabilization (green). After permeabilization, the LCVs were labeled with anti-L. pneumophila (Lpn, red). (B). The LCVs were permeabilized then labeled with anti-AnkB and anti-L. pneumophila antibodies. Samples were analyzed by confocal microscopy and analyses were based on examination of 100 LCVs from different coverslips from triplicate samples. The data are representatives of three independent experiments.
The F-Box and ANK Domains are Dispensable for Anchoring AnkB to the LCV Membrane but are Essential for Biological Function within D. discoideum
Our data above showed that farnesylation was indispensable for targeting AnkB to the plasma membrane of D. discoideum during ectopic expression but that the ANK domains contributed to this localization. Therefore, we examined whether the two ANK domains also contributed to localization of AnkB to the LCV membrane during infection of D. discoideum by L. pneumophila. Semi-purified LCVs from infected D. discoideum harboring the wild type strain of L. pneumophila or its isogenic mutants, were labeled with anti-AnkB antibodies prior to or after permeabilization of membranes, as described previously (Price et al., 2010b). After permeabilization, L. pneumophila was labeled with DAPI. To ensure that isolation of the LCVs did not disrupt their integrity, anti-SidC antibodies were used to label the LCVs, since the SidC effector is localized to the cytosolic face of the LCV membrane (Ragaz et al., 2008). Prior to permeabilization of membranes, anti-AnkB antibodies recognized AnkB on the LCVs that harbor the WT strain, as expected, indicating localization of AnkB to the cytosolic face of the LCV membrane (Figure 6; Price et al., 2010b). Both permeabilized and non-permeabilized LCVs containing the ankB mutant failed to bind the anti-AnkB antibodies (student’s t-test, p < 0.005; Figure 6; Price et al., 2010b). The AnkB-ΔF-box or AnkB-9L10P/AA variant forms of AnkB were also detected on the LCV prior to permeabilization of membranes. Interestingly, the AnkBΔA1, AnkBΔA2, AnkBΔA1A2 variants forms of AnkB were also localized to the cytosolic face of the LCV membrane. This is in contrast to the ectopic expression where the two ANK domains contributed to targeting of AnkB to the plasma membrane (Figure 1). We conclude that the two ANK domains and the F-box domain do not contribute to targeting of AnkB to the LCV membrane within D. discoideum.
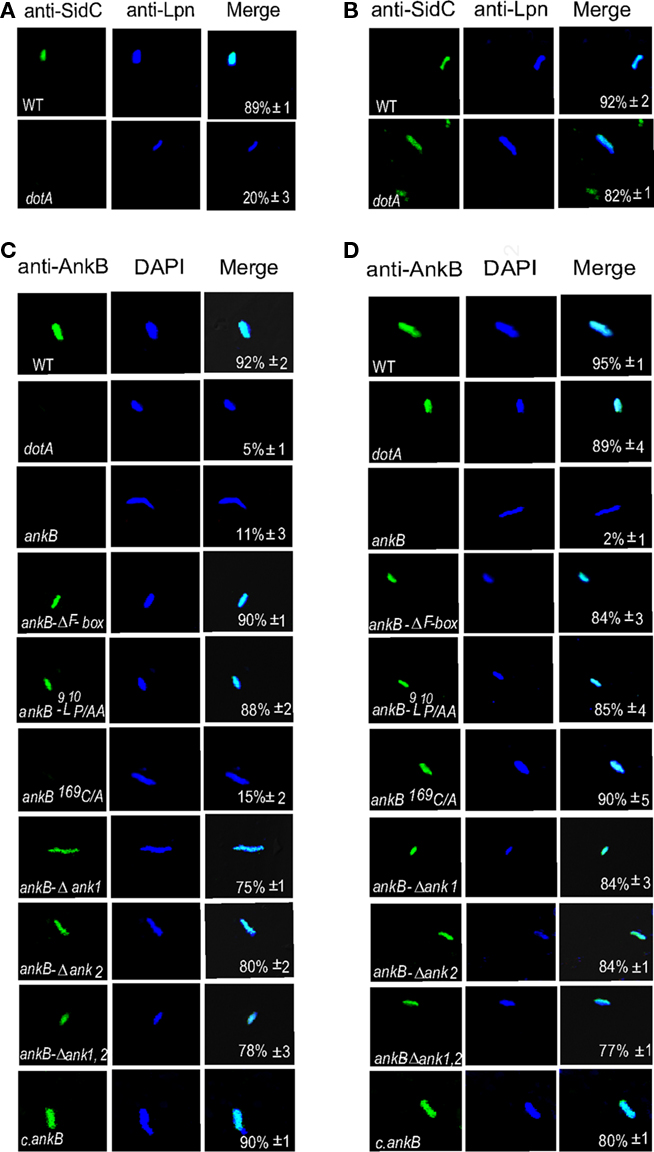
Figure 6. The two ANK domains are dispensable for anchoring AnkB to the cytosolic face of the LCV membrane. The infection was performed for 1 h and the cells were lysed at 2 h post-infection to purify the LCVs. Semi-purified LCVs were analyzed by confocal microscopy. Representative confocal microscopy images that show location of AnkB at the cytosolic face of LCVs. (A) The LCVs were probed with anti-SidC prior to permeabilization of membranes (green). After permeabilization of membranes, the LCVs were stained with DAPI to visualize L. pneumophila (Lpn blue). (B) The LCVs were permeabilized then labeled with anti-SidC and DAPI stain. (C,D) Integrity of the membrane of semi-purified LCVs from D. discoideum was verified by (C) labeling with anti-AnkB antibodies (green) prior to permeabilization of the LCVs. After permeabilization, the LCVs were labeled with anti-Lpn antibodies (blue) within the LCVs. (D) The LCVs were permeabilized followed by labeling with anti-AnkB and anti-Lpn antibodies (blue). Quantification is shown in the merged panels, where the numbers represent the percentage +SD of LCVs that showed localization of AnkB to the cytosolic face of the LCV membrane. Quantitation was based on analyses of 100 LCVs from different coverslips. The data are representatives of three independent experiments.
Although the two eukaryotic-like ANK domains of AnkB were not involved in targeting the effector to the LCV membrane, we examined whether the two domains contributed to the biological function of AnkB in intracellular proliferation of L. pneumophila within ameba. The intracellular growth kinetics analysis was performed in D. discoideum and A. polyphaga. Mutants with in-frame deletions of either or both of the two ANK domains (ankBΔA1, ankBΔA2, and ankBΔA1A2), the wild type strain, or the ankB null mutant were used to infect D. discoideum and A. polyphaga. The complemented ankB mutant and the translocation-defective dotA mutant were used as positive and negative controls, respectively. The cells were infected with MOI of 10 for 1 h. Gentamicin treatment was followed for another hour to kill extracellular bacteria. The data showed that the ankB mutant exhibited a severe intracellular growth defect within D. discoideum and A. polyphaga and the defect was complemented by the native ankB, as expected (Figure 7). The kinetics of the intracellular growth of the ANK domains deletion mutants showed a partial defect in intracellular growth at 24 and 48 h but significant (Student’s t-test, p < 0.05). As expected, the negative control dotA mutant strain did not replicate within D. discoideum or A. polyphaga. Therefore, the ANK domains are not required for targeting AnkB to the cytosolic face of the LCV membrane but they are indispensable for full biological function of AnkB in promoting intracellular proliferation within ameba.
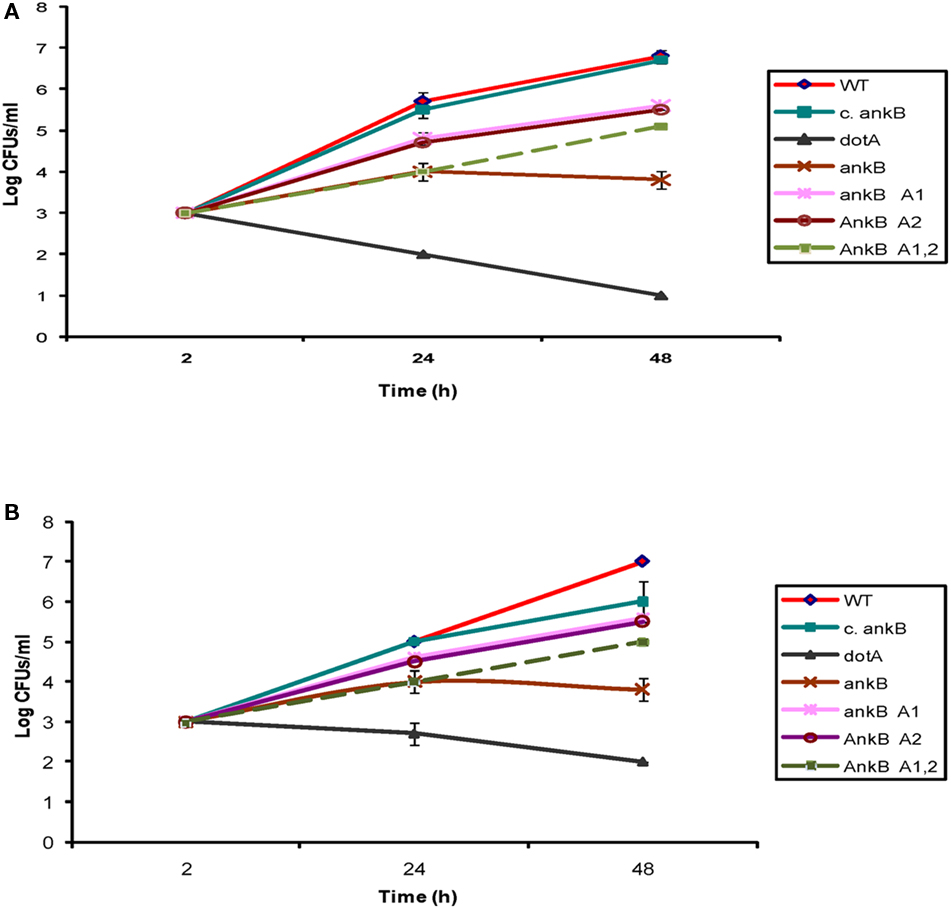
Figure 7. The two ANK domains of AnkB are essential for the biological function of AnkB in intracellular growth of L. pneumophila in D. discoideum. (A) D. discoideum or (B) A. polyphaga were infected with the WT strain, the ankB mutant, or the ankB mutant harboring one of the mutant alleles ankBΔA1, ankBΔA2, or ankBΔA1A2. The ankB mutant complemented with native WT ankB (c.ankB) and the dotA were used as controls. The infection was carried out for 1 h using an MOI of 10 followed by treatment with gentamicin for 1 h. The infected monolayers were lysed at different time points and plated onto agar plates for colony enumeration. The results are representative of three independent experiments performed in duplicate.
DotA/Icm-Dependent Recruitment of FTase RCE-1, and Icmt to the LCV within D. discoideum
The FTase is cytosolic while the other two processing enzymes RCE-1 and Icmt are located in the ER, and the three enzymes are highly conserved through evolution at the structural and functional levels. Since AnkB is only detectable on the LCV during infection of ameba, we hypothesized that ameba-mediated post-translational modification of the effector and its subsequent anchoring to the LCV membrane occurred locally at the ER-derived LCV membrane. To determine whether the three enzymes were recruited to the LCV in ameba, confocal microscopic analyses were performed after 2 h of infection of D. discoideum with the wild type strain AA100, the ΔankB null mutant, the dotA translocation-defective mutant, or the ankB169C/A mutant. Our data showed that 76% of the WT strain-containing LCVs co-localized with the host FTase, and ∼60% co-localized with RCE-1 and Icmt (Figure 8). Importantly, the LCVs that harbored the translocation-defective dotA mutant failed to recruit the three farnesylation enzymes. Interestingly, LCVs that harbor the ΔankB mutant and the ankB169C/A mutant also co-localized with the three host enzymes; FTα, RCE-1, and Icmt. We conclude that the three farnesylation enzymes FTase, RCE-1, and Icmt of D. discoideum are recruited to the LCVs in a Dot/Icm-dependant manner, but AnkB is dispensable for this recruitment.
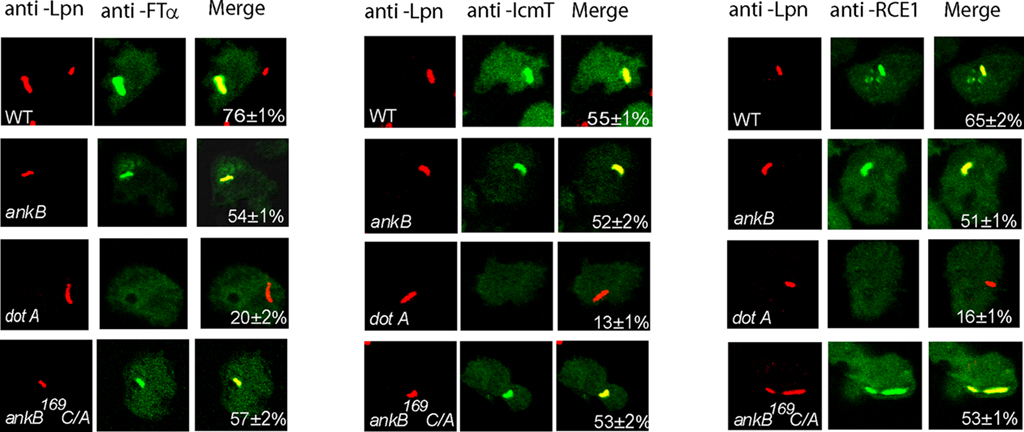
Figure 8. Dot/Icm-dependent recruitment of the three farnesylation enzymes FTα, RCE-1, and Icmt to the LCV within D. discoideum. D. discoideum were infected with various strains for 1 h. At 2 h after infection, the cells were labeled with anti-Lpn antibodies (red) and anti-FTα, anti-RCE-1, or anti-IcmT antibodies (green) and analyzed by confocal microscopy, and analyses were based on examination of 100 LCVs from different coverslips from triplicate samples. The data are representatives of three independent experiments.
Discussion
It has been generally believed that L. pneumophila has evolved through frequent interaction with various protozoa, which has facilitated its infection of mammalian cells. We have previously shown that AnkB is necessary for decorating the LCV with polyubiquitinated proteins and is essential for intracellular proliferation within protozoan hosts, mammalian cells and for intrapulmonary proliferation in the mouse model of Legionnaires’ disease (Al-Khodor et al., 2008; Habyarimana et al., 2008; Price et al., 2009). The AnkB effector is the first remarkable example of how L. pneumophila exploits conserved eukaryotic processes, which are the ubiquitination and farnesylation machineries to proliferate within the two evolutionarily distant hosts, mammalian and protozoan cells (Price et al., 2009, 2010a,b; Al-Khodor et al., 2010; Price and Abu Kwaik, 2010).
Our data show that the ankB mutant that is defective in intracellular proliferation is trans-rescued for its defect within D. discoideum ectopically expressing AnkB that is biologically functional as platforms for the docking of polyubiquitinated proteins to the plasma membrane of D. discoideum. This is the first demonstration of trans-rescue of a L. pneumophila mutant in ameba by ectopic expression of the lost protein. Remarkably, similar phenomenon is also exhibited in human-derived cells (Price et al., 2009). It is unclear why ectopically expressed AnkB is targeted to the plasma membrane but not at the LCV. The mechanism by which this trans-rescue occurs is not known. We speculate that it is possible that the host factors ubiquitinated by AnkB on the LCV are also ubiquitinated during ectopic expression of AnkB, which would ensure formation of a replicative niche, but we find that to be unlikely. It is important to note that ectopic expression is an artificial system that may not represent what is exhibited during infection as the case in here. Interestingly, during ectopic expression in D. discoideum the two ANK domains contribute to localization of AnkB to the plasma membrane and are required for polyubiquitination. The two ANK domains of AnkB are also required for the recruitment of polyubiquitinated proteins to the plasma membrane during ectopic expression in D. discoideum. However, during infection, the two ANK domains are dispensable for localization of AnkB to cytosolic face of the LCV membrane. It is possible that the ANK domains interact with host cell targets that are located in the plasma membrane, which may be supported by recent work that the AnkB allele of the Paris strain of L. pneumophila interacts with Parvin B located in the plasma membrane (Lomma et al., 2010).
Our data show that the F-box domain of AnkB interacts specifically with the Skp1 protein the component of the SCF1 ubiquitin ligase complex of D. discoideum, similar to mammalian cells. Other studies have shown that orthologs of AnkB in the Philadelphia (legU13) and Paris (lpp2082) strains of L. pneumophila interact with mammalian Skp1 (Ensminger and Isberg, 2010; Lomma et al., 2010). Further investigations are needed to verify the interaction of AnkB with other components of the SCF1 complex and identify the substrates that are polyubiquitinated.
During infection by L. pneumophila, AnkB is modified by the farnesylation machinery of D. discoideum and this post-translation modification of the microbial effector is essential for anchoring AnkB to the LCV membrane, which is indispensable for the biological function of the effector. Remarkably, the three host enzymes (FTase, RCE-1, and IcmT) that constitute the farnesylation enzymatic machinery are recruited to the LCV within D. discoideum by a Dot/Icm-dependent process but AnkB is dispensable for this recruitment. Since IcmT and RCE-1 are localized to the ER and the LCV is ER-derived, we postulate that these two enzymes are part of the LCV membrane that is derived from the ER in a Dot/Icm-dependent manner. Since farnesyl transferase is cytosolic and its recruitment is Dot/Icm-dependent, it is likely that another Dot/Icm-translocated effector(s) of L. pneumophila is involved in recruiting this host cytosolic enzyme to the LCV membrane. It is likely that recruitment of the three farnesylation enzymes would ensure local post-translational farnesylation and processing of the C-terminus of AnkB to anchor the effector to the LCV membrane, without exporting AnkB to the cytosol where it may disrupt various cellular membranes. This compartmentalized hijacking of the polyubiquitination and farnesylation machineries by the LCV is likely to be a major factor in the success of this pathogen to proliferate intracellularly while maintaining a viable host cell that can sustain intracellular proliferation of the pathogen.
The AnkB microbial effector that is injected into the host cell is mostly composed of eukaryotic-like domains that include an F-box domain and two ANK domains, in addition to a C-terminal eukaryotic CaaX motif (Al-Khodor et al., 2010; Price et al., 2010b). The three eukaryotic domains of AnkB are essential for the biological function of AnkB in intracellular proliferation of L. pneumophila within D. discoideum, similar to mammalian cells. We propose that it is more likely that this effector has been acquired by L. pneumophila through inter-kingdom horizontal gene transfer from a primitive unicellular or multicellular eukaryotic host (Al-Khodor et al., 2010). This hypothesis is supported by the domain architecture of this F-box effector that resembles those of F-box proteins of unicellular eukaryotes where ANK domains constitute the protein–protein interaction domains that determine specificity of F-box proteins (Al-Khodor et al., 2010, Price and Abu Kwaik, 2010). In contrast, mammalian F-box proteins do not have ANK domains, but do have WD or LRR as protein–protein interaction domains, instead. However, convergent evolution of AnkB may not be excluded at this time. It is interesting that the ANK domains are also essential for intracellular proliferation of L. pneumophila within evolutionarily distant host cells, human macrophages, and ameba. It would be interesting to identify the host substrates that bind the ANK domains within the two evolutionarily distant hosts. Based on our findings, we speculate that these substrates are likely to be evolutionarily conserved.
In summary, our data show the hijacking of two evolutionarily conserved eukaryotic processes by the AnkB effector and the remarkable similarities in the molecular and biochemical events orchestrated in evolutionarily distant eukaryotic hosts. We propose that such hijacking of evolutionarily conserved eukaryotic machineries through inter-kingdom horizontal gene transfer of the F-box effector from primitive eukaryotes to L. pneumophila is a factor in the ability of this organism to proliferate within human macrophages and the emergence of Legionnaires’ disease in humans. However, the pulmonary tissue tropism of L. pneumophila and its exploitation of pro- and anti-apoptotic processes of higher eukaryotes (Amer, 2010) suggest additional processes are involved in the evolution of this human pathogen.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Yousef Abu Kwaik is supported by Public Health Service Awards R01AI43965 and R01AI069321 from NIAID and by the commonwealth of Kentucky Research Challenge Trust Fund. We would like to thank Dictyostelium stock center for providing us with AX2 strain and Dictyostelium plasmids.
References
Al-Khodor, S., Price, C. T., Habyarimana, F., Kalia, A., and Abu Kwaik, Y. (2008). A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa. Mol. Microbiol. 70, 908–923.
Al-Khodor, S., Price, C. T., Kalia, A., and Abu Kwaik, Y. (2010). Functional diversity of ankyrin repeats in microbial proteins. Trends Microbiol. 18, 132–139.
Amer, A. O. (2010). Modulation of caspases and their non-apoptotic functions by Legionella pneumophila. Cell. Microbiol. 12, 140–147.
Berger, K. H., and Isberg, R. R. (1993). Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7, 7–19.
Bergo, M. O., Leung, G. K., Ambroziak, P., Otto, J. C., Casey, P. J., and Young, S. G. (2000). Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J. Biol. Chem. 275, 17605–17610.
Bitar, D. M., Molmeret, M., and Abu Kwaik, Y. (2004). Molecular and cell biology of Legionella pneumophila. Int. J. Med. Microbiol. 293, 519–527.
Boyartchuk, V. L., Ashby, M. N., and Rine, J. (1997). Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275, 1796–1800.
Clarke, M., Bazari, W. L., and Kayman, S. C. (1980). Isolation and properties of calmodulin from Dictyostelium discoideum. J. Bacteriol. 141, 397–400.
Dai, Q., Choy, E., Chiu, V., Romano, J., Slivka, S. R., Steitz, S. A., Michaelis, S., and Philips, M. R. (1998). Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J. Biol. Chem. 273, 15030–15034.
Dorer, M. S., Kirton, D., Bader, J. S., and Isberg, R. R. (2006). RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2, e34. doi: 10.1371/journal.ppat.0020034
Ensminger, A. W., and Isberg, R. R. (2010). E3 ubiquitin ligase activity and targeting of BAT3 by multiple Legionella pneumophila translocated substrates. Infect. Immun. doi: 10.1128/IAI.00344-10. [Epub ahead of print].
Fields, B. S., Benson, R. F., and Besser, R. E. (2002). Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 15, 506–526.
Fields, H. S. (1996). Establishing core performance requirements for automated TPN compounders. Am. J. Health Syst. Pharm. 53, 1607–1608.
Habyarimana, F., Al-Khodor, S., Kalia, A., Graham, J. E., Price, C. T., Garcia, M. T., and Abu Kwaik, Y. (2008). Role for the Ankyrin eukaryotic-like genes of Legionella pneumophila in parasitism of protozoan hosts and human macrophages. Environ. Microbiol. 10, 1460–1474.
Harb, O. S., Gao, L. Y., and Abu Kwaik, Y. (2000). From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ. Microbiol. 2, 251–265.
Hu, C. D., Chinenov, Y., and Kerppola, T. K. (2002). Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9, 789–798.
Isberg, R. R., O’Connor, T. J., and Heidtman, M. (2009). The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24.
Kaufmann, A. F., McDade, J. E., Patton, C. M., Bennett, J. V., Skaliy, P., Feeley, J. C., Anderson, D. C., Potter, M. E., Newhouse, V. F., Gregg, M. B., and Brachman, P. S. (1981). Pontiac fever: isolation of the etiologic agent (Legionella pneumophilia) and demonstration of its mode of transmission. Am. J. Epidemiol. 114, 337–347.
Kerscher, O., Felberbaum, R., and Hochstrasser, M. (2006). Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22, 159–180.
Knetsch, M. L. W., Tsiavaliaris, G., Zimmermann, S., Rühl, U., and Manstein, D. J. (2002). Expression vectors for studying cytoskeletal proteins in Dictyostelium discoideum. J. Muscle Res. Cell. Motil. 23, 605–611.
Lerner, E. C., Qian, Y., Blaskovich, M. A., Fossum, R. D., Vogt, A., Sun, J., Cox, A. D., Der, C. J., Hamilton, A. D., and Sebti, S. M. (1995). Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J. Biol. Chem. 270, 26802–26806.
Lomma, M., Dervins-Ravault, D., Rolando, M., Nora, T., Newton, H. J., Samson, F. M., Sahr, T., Gomez-Valero, L., Jules, M., Hartland, E. L., and Buchrieser, C. (2010). The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell. Microbiol. 12, 1272–1291.
Molmeret, M., Bitar, D. M., Han, L., and Abu Kwaik, Y. (2004). Disruption of the phagosomal membrane and egress of Legionella pneumophila into the cytoplasm during the last stages of intracellular infection of macrophages and Acanthamoeba polyphaga. Infect. Immun. 72, 4040–4051.
Molmeret, M., Horn, M., Wagner, M., Santic, M., and Abu Kwaik, Y. (2005). Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71, 20–28.
Molmeret, M., Jones, S., Santic, M., Habyarimana, F., Esteban, M. T., and Abu Kwaik, Y. (2010). Temporal and spatial trigger of post-exponential virulence-associated regulatory cascades by Legionella pneumophila after bacterial escape into the host cell cytosol. Environ. Microbiol. 12, 704–715.
Pang, K. M., Lynes, M. A., and Knecht, D. A. (1999). Variables controlling the expression level of exogenous genes in Dictyostelium. Plasmid 41, 187–197.
Price, C. T., Al-Khodor, S., Al-Quadan, T., and Abu Kwaik, Y. (2010a). Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae. Infect. Immun. 78, 2079–2088.
Price, C. T., Al-Quadan, T., Santic, M., Jones, S. C., and Abu Kwaik, Y. (2010b). Exploitation of conserved eukaryotic host cell farnesylation machinery by an F-box effector of Legionella pneumophila. J. Exp. Med. 207, 1713–1726.
Price, C. T., Al-Khodor, S., Al-Quadan, T., Santic, M., Habyarimana, F., Kalia, A., and Abu Kwaik, Y. (2009). Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog. 5, e1000704. doi: 10.1371/journal.ppat.1000704
Price, C. T. D., and Abu Kwaik, Y. (2010). Exploitation of host polyubiquitination machinery through molecular mimicry by eukaryotic-like bacterial F-box effectors. Front. Microbiol. 1:122. doi: 10.3389/fmicb.2010.00122
Purcell, M., and Shuman, H. A. (1998). The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect. Immun. 66, 2245–2255.
Ragaz, C., Pietsch, H., Urwyler, S., Tiaden, A., Weber, S. S., and Hilbi, H. (2008). The Legionella pneumophila phosphatidylinositol-4 phosphate- binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell. Microbiol. 10, 2416–2433.
Solomon, J. M., Rupper, A., Cardelli, J. A., and Isberg, R. R. (2000). Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host- pathogen interactions. Infect. Immun. 68, 2939–2947.
Swanson, M. S., and Hammer, B. K. (2000). Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54, 567–613.
Veltman, D. M., Akar, G., Bosgraaf, L., and Van Haastert, P. J. (2009). A new set of small, extrachromosomal expression vectors for Dictyostelium discoideum. Plasmid 61, 110–118.
Vogel, J. P., Andrews, H. L., Wong, S. K., and Isberg, R. R. (1998). Conjugative transfer by the virulence system of Legionella pneumophila. Science 279, 873–876.
Wright, L. P., and Philips, M. R. (2006). Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J. Lipid Res. 47, 883–891.
Keywords: dot/Icm, Skp1, farnesyl, SCF1, prenylation
Citation: Al-Quadan T and Abu Kwaik Y (2011) Molecular characterization of exploitation of the polyubiquitination and farnesylation machineries of Dictyostelium discoideum by the AnkB F-box effector of Legionella pneumophila. Front. Microbio. 2:23. doi: 10.3389/fmicb.2011.00023
Received: 13 January 2011;
Paper pending published: 25 January 2011;
Accepted: 31 January 2011;
Published online: 14 February 2011.
Edited by:
Amal Amer, The Ohio State University, USAReviewed by:
Dario S. Zamboni, Universidade de São Paulo, BrazilDaniel E. Voth, University of Arkansas for Medical Sciences, USA
Joana Costa, Center for Neuroscience and Cellular Biology, Portugal
Copyright: © 2011 Al-Quadan and Abu Kwaik. This is an open-access article subject to an exclusive license agreement between the authors and Frontiers Media SA, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Yousef Abu Kwaik, Department of Microbiology and Immunology, College of Medicine, University of Louisville, Louisville, KY 40292, USA. e-mail: abukwaik@louisville.edu

