Pattern formation during development of the embryonic cerebellum
- 1Faculty of Medicine, Department of Cell Biology and Anatomy, Genes and Development Research Group, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
- 2Division of Neuroscience, San Raffaele Scientific Institute, Milan, Italy
The patterning of the embryonic cerebellum is vital to establish the elaborate zone and stripe architecture of the adult. This review considers early stages in cerebellar Purkinje cell patterning, from the organization of the ventricular zone to the development of Purkinje cell clusters—the precursors of the adult stripes.
Introduction—Patterning of the Adult Cerebellum
The adult mouse cerebellum houses a complex topographical map (e.g., Figure 1). The map probably involves all cell types in the cerebellar cortex (e.g., Purkinje cells—Hawkes et al., 1985; granule cells—Hawkes and Turner, 1994; Hawkes et al., 1998; Golgi cells—Sillitoe et al., 2008; basket cells—Demilly et al., 2011; glia—Scott, 1963): this review is focused on the Purkinje cells. The mapping of Purkinje cell antigens (e.g., zebrin II/aldolase c—Brochu et al., 1990; phospholipase (PL)Cβ4—Sarna et al., 2006; heat shock protein (HSP)25—Armstrong et al., 2000; CART—Reeber and Sillitoe, 2011 etc.), gene transcripts (reviewed in Sillitoe and Joyner, 2007) and transgenes (e.g., L7/pcp2-lacZ—Vandaele et al., 1991; Oberdick et al., 1993; OMP-lacZ—Nunzi et al., 1999; IP3R1-nls-lacZ—Furutama et al., 2010 etc.), has revealed multiple Purkinje cell subtypes. Each subtype has a characteristic distribution, but it seems plausible that these are all reflections of a common underlying architecture (Apps and Hawkes, 2009). First, the cerebellar cortex is divided from anterior to posterior into transverse zones: the anterior zone (AZ: ∼lobules I–V), the central zone (CZ: ∼lobules VI–VII; possibly further subdivided—Marzban et al., 2008), the posterior zone (PZ: ∼lobules VIII-dorsal IX) and the nodular zone (NZ: ∼lobules IX ventral and X: Ozol et al., 1999; Sillitoe and Hawkes, 2002). Next, each transverse zone is divided mediolaterally into parasagittal stripes. The most broadly studied marker of adult stripes is the Purkinje cell antigen zebrin II/aldolase C (e.g., Brochu et al., 1990; Ahn et al., 1994). The opposite pattern is revealed by other markers, for example PLCβ4 (Sarna et al., 2006) and EBF2 (Croci et al., 2006; Chung et al., 2008). The zone-and-stripe pattern is highly reproducible between individuals and conserved across mammals and birds: zebrin II is expressed by many vertebrates (e.g., fish—Lannoo et al., 1991a,b; Meek et al., 1992), as is EBF2 (Malgaretti et al., 1997; Bally-Cuif et al., 1998; Dubois and Vincent, 2001), but arrays of stripes are only seen in birds (e.g., pigeon—Pakan et al., 2007; chicken—Marzban et al., 2010) and mammals (reviewed in Sillitoe et al., 2005; Marzban and Hawkes, 2011). Many molecular markers are co-localized with either the zebrin II+ or zebrin II– Purkinje cells (e.g., PLCβ3—Sarna et al., 2006; sphingosine kinase 1a—Terada et al., 2004 etc.). However, this is not the extent of the stripe compartmentation—other markers reveal subdivisions within stripes, subsets of stripes within the zebrin II+/– sets, and stripes in the CZ and NZ (e.g., P-path—Leclerc et al., 1992; heat shock protein (HSP)25—Armstrong et al., 2000; human natural killer cell antigen 1 (HNK1)—Eisenman and Hawkes, 1993; Marzban et al., 2004). In sum, the adult cerebellar cortex is highly reproducibly subdivided into several hundred distinct modules with >10 distinct Purkinje cell phenotypes (e.g., reviewed in Hawkes and Gravel, 1991; Hawkes, 1997; Apps and Hawkes, 2009). In the mouse, a typical stripe/module comprises fewer than a thousand Purkinje cells.

Figure 1. Zones and stripes in the adult mouse cerebellum. Anti-zebrin II staining of a whole mount, adult mouse cerebellum reveals an elaborate pattern of Purkinje cell stripes (for method and more details—see Sillitoe and Hawkes, 2002).
Zones and stripes are important because cerebellar patterning influences all aspects of cerebellar organization and function. Here is not the place to expound this at length, but simply to note that the Purkinje cell map serves as a scaffold around which many other cerebellar structures are organized:
- Afferent projections use Purkinje cells to target their terminal fields.
- Interneurons are restricted at stripe boundaries and are thought to use Purkinje cell cues to establish their own topography.
- Functional boundaries align with stripe boundaries (e.g., Chockkan and Hawkes, 1994; Chen et al., 1996; Hallem et al., 1999; Apps and Garwicz, 2005; Wadiche and Jahr, 2005).
- Specific target zones in the cerebellar and vestibular nuclei receive topographically ordered projections from stripes in the cerebellar cortex (e.g., Hawkes and Leclerc, 1986; Chung et al., 2009a; Sugihara, 2011).
- Cerebellar mutant phenotypes are frequently restricted at zone or stripe expression boundaries (e.g., Eisenman, 2000; Beirebach et al., 2001).
- Purkinje cell death due to mutation or insult is typically restricted to parasagittal stripes (e.g., reviewed in Sarna and Hawkes, 2003).
Thus, Purkinje cell stripes lie at the heart of cerebellar structure, function, and pathology. How does this remarkable pattern develop? Where do Purkinje cell subtypes come from? How do they end up in stripes?
Cerebellar pattern formation is conventionally divided into four broad stages (the timings refer to the mouse cerebellum: Figure 2):
- The formation of the cerebellar ventricular zone (∼E7–E10 in mouse);
- Purkinje cell birth in the ventricular zone and Purkinje cell subtype specification (∼E10–E13);
- Purkinje cell migration from the SVZ and reorganization into a stereotyped embryonic cluster array (∼E14–E17); and
- Purkinje cell cluster dispersal and refinement to form the adult stripes (∼E18–P20).
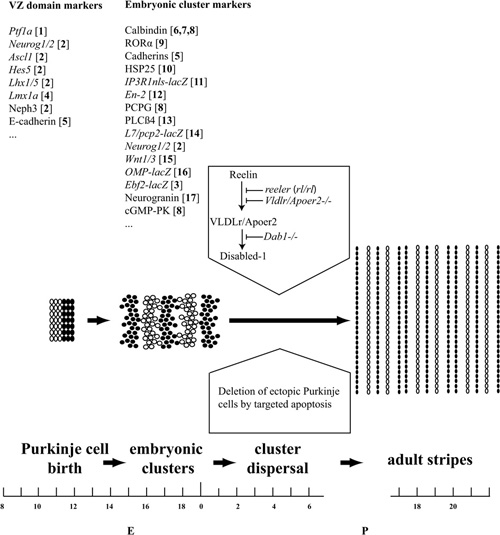
Figure 2. Pattern formation in the mouse cerebellum. Cerebellar pattern formation is divided here into three broad stages: Purkinje cell birth in the ventricular zone and Purkinje cell subtype specification (∼E10–E14); Purkinje cell reorganization into a stereotyped embryonic cluster array (∼E14–E18); and cluster dispersal and refinement to form the adult stripes (∼E18–P20). Examples of markers that reveal expression domains in the VZ and early Purkinje cell clusters are listed above. References: [1] Hoshino et al. (2005); [2] Zordan et al. (2008); [3] Croci et al. (2006); [4] Chizhikov and Millen (2003); [5] Redies et al. (2011); [6] Tano et al. (1992); [7] Wassef et al. (1990); [8] Wassef et al. (1985); [9] Sillitoe et al. (2009); [10] Armstrong et al. (2001); [11] Furutama et al. (2010); [12] Millen et al. (1995); [13] Marzban et al. (2007); [14] Ozol et al. (1999); [15] Bally-Cuif et al. (1992); [16] Nunzi et al. (1999); [17] Larouche et al. (2006). Substantial Purkinje cell death occurs by apoptosis in the first postnatal week, and we hypothesize that this may play a role in refining the cerebellar map. The transformation of Purkinje cell clusters into stripes is triggered by Reelin binding to a Very Low Density Lipoprotein receptor/Apolipoprotein receptor 2 complex (Vldlr/Apoer2), which leads to tyrosine phosphorylation of the docking protein Dab1. These events are blocked by mutations of Reelin production (reeler), the Reelin receptors (Apoer2/Vldlr double null), or downstream signals (Dab1–/–).
This review is focused on stages 2 and 3—Purkinje cell subtype specification and the early stages of pattern formation. We also focus this review on patterning of Purkinje cells—less is known of the development of patterns in either granule cells or inhibitory interneurons although many of these are thought to be secondary to the patterning of the Purkinje cells (e.g., Sotelo and Chédotal, 2005; Sillitoe et al., 2008, 2010; Chung et al., 2009a,b).
The Structure of the Ventricular Zone
The cerebellar primordium arises from the rostral metencephalon between E8.5–E9.5 (e.g., Wassef and Joyner, 1997; Sillitoe and Joyner, 2007). It houses two distinct germinal matrices, the dorsal rhombic lip and the ventral ventricular zone (VZ) of the fourth ventricle, which generate neuronal precursors fated to adopt GABAergic and glutamatergic phenotypes respectively (Figure 3). The earliest stage of cerebellar development depends on fibroblast growth factor 8 (FGF8) secreted by the isthmic organizer (Crossley et al., 1996; Joyner, 1996; Liu et al., 1999; Martinez et al., 1999) at the midbrain-hindbrain boundary. Mutant mice with reduced Fgf8 expression have defective cerebellar development, and ectopic FGF8 expression leads to ectopic cerebellar tissues (reviewed in Nakamura et al., 2008). FGF8 secretion initiates the expression of multiple region-specific transcription factors, including EN1/2, PAX2/5/8, OTX2, and GBX2. In addition, FGF8 initiates Wnt1 expression at the midbrain-hindbrain boundary, and FGF8 and WNT1 form a positive feedback loop, which also involves EN2 and PAX2 (Thomas and Capecchi, 1990; McMahon et al., 1992; Bally-Cuif et al., 1992; Millen et al., 1995; Martinez et al., 1999; Simeone, 2000), and together act as organizers to pattern the tissues around the midbrain-hindbrain boundary.
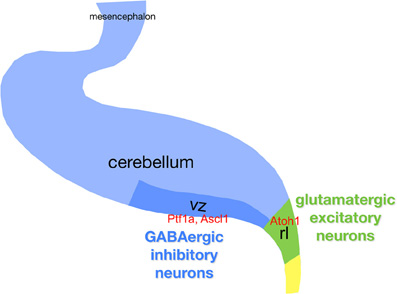
Figure 3. Schematic of the cerebellar primordium with respect to neurogenesis. Gene names are in red boldface. Abbreviations: ctz, cortical transitory zone; egl, external granule layer; ntz, nuclear transitory zone; rl, upper rhombic lip; rp, roof plate; vz, ventricular zone.
Although the cerebellum contains a relatively small variety of neurons, the molecular machinery governing neuronal generation and/or subtype specification is still poorly understood. Genetic fate mapping shows that a Ptf1a- (pancreas transcription factor 1a—which encodes a bHLH transcription factor) expressing domain in the VZ gives rise to all Purkinje cells (Hoshino et al., 2005; Hoshino, 2006). In 2005, the characterization of a novel mutant mouse, cerebelless, which lacks the entire cerebellar cortex but survives into adulthood, was reported (Hoshino et al., 2005). The analysis of the phenotype, and the characterization of the underlying gene mutation, clarified that PTF1A is required for generating all cerebellar GABAergic compartment. ATOH1 and PTF1A participate in regionalizing the cerebellar neuroepithelium, and define two distinct areas, the VZ (Ptf1a) and the upper rhombic lip (Atoh1), which generate GABAergic and glutamatergic neurons, respectively (Hoshino et al., 2005; Pascual et al., 2007). In regard to GABAergic progenitors Purkinje cells are distinguished from interneurons by differential expression of E-cadherin in cycling progenitors (Mizuhara et al., 2010) and of the transcriptional corepressor Corl2 (Minaki et al., 2008) in post-mitotic precursors, while two other Ptf1a targets (Nephrin and Neph3) (Nishida et al., 2010) are expressed by all GABA progenitors. Moreover, the expression domains of three proneural genes (Ascl1, Neurog1, and Neurog2) overlap with that of Ptf1a in the VZ.
While many studies have investigated the roles played by ATOH1 in establishing the cerebellar glutamatergic lineage, fewer studies have explored GABAergic precursors born in the cerebellar VZ. In 2008, Zordan et al. published a systematic descriptive analysis of proneural gene expression at early stages of mouse cerebellar development (Figure 4). This established that at the onset of cerebellar neurogenesis (∼E11), the Ascl1 transcript becomes detectable in the VZ and presumptive NTZ. A similar distribution is observed at later stages, with the Ascl1 transcript occupying the entire thickness of the Ptf1a+ VZ all the way to its apical (ventricular) margin. Accordingly, the territories occupied by Ascl1 and Atoh1 are clearly complementary. Ascl1 remains confined to the VZ until E13.5. An additional study by Johnson and coworkers (Kim et al., 2011) described genetic fate mapping studies done by using two transgenic Ascl1-Cre lines, one of which expressed a tamoxifen-inducible Cre recombinase, CreERTM (Helms et al., 2005; Battiste et al., 2007) and two Cre-inducible reporter lines (Soriano, 1999; Srinivas et al., 2001). The evidence produced in this elegant lineage analysis study is in full agreement with Zordan et al. In particular, Ascl1+ progenitors are initially (E12.5) restricted to the cerebellar VZ and excluded both from the post-mitotic cerebellar transitory zone and from the rhombic lip migratory stream. The Ascl1+ and Atoh1+ progenitor domains are mutually exclusive, whereas a high degree of overlap exists between Ptf1a+ and Ascl1+ progenitors, suggesting that Ascl1 labels GABAergic neuronal progenitors. However, by E17.5 Ascl1+ progenitors are no longer confined to the VZ but are also found scattered throughout the cerebellar primordium.
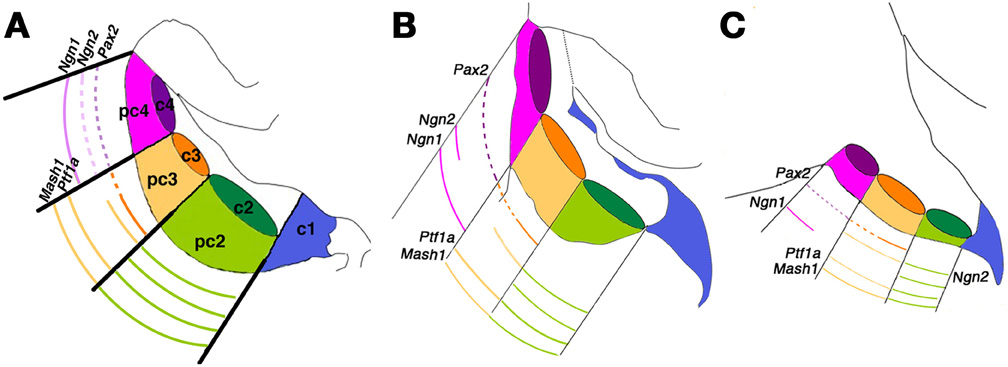
Figure 4. Patterning the cerebellar ventricular zone. An integrated molecular map of embryonic day (E) 12.5 cerebellar primordium populations. This model attempts to integrate the results of Chizhikov et al. (2006) with those of Zordan et al. (2008) to provide a three-dimensional view of gene expression. (A–C) Representative sagittal sections spanning the mediolateral axis of the embryonic cerebellum at E12.5: (A) medial; (B) intermediate; (C) lateral. All sketches contain a representation of the three mitotic progenitor domains (pc2–4) and post-mitotic precursor domains (c2–4), in addition to the rhombic lip (c1). Solid lines indicate robust expression levels; dashed lines indicate lower transcript levels. Adapted from Zordan et al. (2008).
Finally, a study by Wassef and co-workers (Grimaldi et al., 2009) further refined the analysis of the role of Ascl1 in cerebellar neurogenesis, incorporating the effects of Ascl1 gene disruption and overexpression. They established that Ascl1+ progenitors progressively delaminate out of the VZ to settle first in the prospective white matter, and then in the cerebellar cortex. By studying an Ascl1-GFP transgenic mouse, they demonstrated that Ascl1+ progenitors give rise to PAX2+ interneurons and OLIG2+ oligodendrocyte precursors, while glutamatergic neurons, astrocytes and Bergmann glial cells did not express GFP. In contrast, the loss of Ascl1 led to a dramatic reduction of PAX2+ and OLIG2+ precursors. No change was found in Purkinje cell development.
Finally, a gain-of-function approach by using in vivo electroporation of a GFP plasmid at E14.5, concluded that most Ascl1+ oligodendrocytes do not originate from the cerebellar VZ. In addition, an Ascl1 plasmid electroporated into the cerebellar VZ led to an increased number of PAX2+ interneurons, fewer OLIG2+ oligodendrocyte precursors, and the complete absence of astroglia. This suggests that Ascl1 overexpression pushes progenitors toward a (PAX2+) interneuron fate and suppresses the astrocytic fate.
Taken together, evidence suggests that Ascl1 contributes to GABAergic interneuron and cerebellar nuclear neuron generation, and to Purkinje cell development. However, it is not required for Purkinje cell specification.
Possible Roles for Neurogenins in the Development of Cerebellar GABAergic Neurons
Neurog1 and Neurog2 are expressed in the Ptf1a+ ventricular neuroepithelium. As shown by Zordan et al. (2008), the Neurog2 transcript is first observed around E11 in cerebellar nuclear neuron progenitors of the cerebellar primordium, whereas Neurog1 appears 1 day later, in a rostral region located between the isthmic organizer, labeled by Fgf8, and the territory marked by Ascl1. At E12.5, both Neurog1 (see also Salsano et al., 2007) and Neurog2 are present in the VZ but with a few differences in distribution: in the anterior cerebellum, Neurog1 is expressed at high levels in a region close to the midline, whereas Neurog2 is restricted to the lateral VZ. In posterior territories, the expression patterns overlap completely. Neurog1 and Neurog2 are adjacent to, and partially overlap with, post-mitotic domains labeled by Lhx1 and Lhx5, two genes that control Purkinje cell differentiation (Zhao et al., 2007). This suggests that Neurog1 and Neurog2 are expressed in progenitors that are undertaking the last cycle of cell division to become post-mitotic Purkinje cell precursors. At E13.5 the differential anterior boundaries of Neurog1 and Neurog2 are maintained, although the transcript levels of both genes are down-regulated. The authors conclude that Neurog1 and Neurog2 are mainly expressed in the cerebellar germinal epithelium that gives rise to GABAergic progenitors, while they are completely absent from the rhombic lip, the source of all glutamatergic cerebellar progenitors. Moreover, their expression patterns are similar but not totally overlapping, suggesting that these two closely related genes may contribute to the diversity of cerebellar GABAergic neurons and, possibly, Purkinje cell subtypes.
Neurog1 is Expressed in Cerebellar GABAergic Interneuron Progenitors
In 2009, Doughty and coworkers published a lineage analysis study that described the mature cerebellar neurons deriving from Neurog1+ cell fates in the developing mouse cerebellum (Lundell et al., 2009). They confirmed the findings of Zordan et al. (2008) and extended the analysis to late embryonic and postnatal cerebellar development. At E14–E20, Neurog1 is present in Ptf1a+ neurons, but it is excluded from the upper rhombic lip and external granular layer. Moreover, at P7, it co-localizes with Ptf1a and BrdU in the deep white matter. This suggests that Neurog1 is expressed in early GABAergic interneuron precursors that, shortly after birth, migrate from the white matter to reach their final destination in the cortex. By using two artificial chromosome (BAC)-reporter mice they analyzed short-term and long-term Neurog1+ cell fates. Neurog1 is expressed in PAX2+ interneuron progenitors but it does not contribute to the GABAergic neuron lineage in the cerebellar nuclei (Vue et al., 2007). Surprisingly, they did not reveal any fluorescence in Purkinje cells. Furthermore, the authors bred Neurog1-Cre transgenic mice into the double reporter Z/EG line (Novak et al., 2000). Z/EG mice express a LacZ cassette under control of a CMV enhancer/chicken actin promoter (pCAGGS). In the presence of a Cre recombinase the lacZ cassette is excised, leading to the activation of the downstream EGFP gene. By using this approach, they revealed scattered GFP+ Purkinje cell neurons, mostly in the hemispheres. Surprisingly, this strategy failed to tag GABAergic interneurons, perhaps due to epigenetic/positional silencing of the reporter transgene or to low-level expression of the Neurog1-Cre transgene. A recent study confirmed the notion that, in the cerebellar primordium, the Neurog1+ lineage contributes to the Purkinje cell pool (Kim et al., 2011).
In summary, Neurog1 is expressed in progenitors giving rise to GABAergic interneurons of the cerebellar cortex and at least some Purkinje cells. However, it does not seem to contribute to the development of neurons of the cerebellar nuclei. While both neurogenin genes are expressed in the cerebellar VZ in presumptive GABAergic neurons, nothing can be inferred to date as regards their function(s). Do they affect cell type or subtype specification, or neuronal vs. glial commitment? And in either case, do they act redundantly with each other or with Ascl1? There seems to be some degree of selectivity, in that Neurog1 is expressed in only a share of GABAergic progenitors and, as predicted by Zordan et al. (2008), the broader expression domain exhibited by Neurog2, a direct PTF1A target gene (Henke et al., 2009), suggests that it may play a unique role in the development of GABAergic cerebellar nuclear neurons. No mechanism has been identified but a recent study of gene expression in the cerebellar primordium of E11.5 Neurog1 null mice suggests that Neurog1 and Pax6 may interact functionally in the activation of downstream targets (Dalgard et al., 2011).
Purkinje Cell Birth and the Formation of the First Laminar Phase
Purkinje cells undergo terminal mitosis in the VZ between E10–E13 in the mouse (Miale and Sidman, 1961; Hashimoto and Mikoshiba, 2003; Namba et al., 2011: Figure 2). Birthdating studies, using incorporation of either adenovirus (Hashimoto and Mikoshiba, 2003) or bromodeoxyuridine (e.g., chick—Karam et al., 2000; mouse—Larouche and Hawkes, 2006), reveal a direct correlation between the birthdate of a Purkinje cell and its final mediolateral location, suggesting that Purkinje cells acquire positional information at or shortly after their terminal differentiation in the VZ. It is not known whether positional information and phenotype are specified at the same time. Post-mitotic Purkinje cells migrate dorsally out of the VZ, in part along radial glia processes (e.g., Morales and Hatten, 2006), and stack in the cerebellar anlage with the earliest-born Purkinje cells located most dorsally.
Specification of Purkinje Cell Subtypes
In many areas of the CNS, the development of patterning is driven by neuronal activity (e.g., the retinotectal system—reviewed in Ruthazer and Cline, 2004), In contrast, Purkinje cell phenotype specification and stripe formation seem to be activity-independent. Experiments both in vivo and in vitro suggest that the zebrin phenotype is specified early in development and is not influenced by subsequent interactions with cerebellar afferents, cerebellar neurons or glia (Leclerc et al., 1988; Wassef et al., 1990; Seil et al., 1995). For example, Purkinje cells in P0 cerebellar slice cultures express both zebrin+/− phenotypes, and blocking neuronal activity or depleting granule cells and glia did not change this (Seil et al., 1995). Next, deafferentation of the neonatal or adult cerebellum does not alter the fundamental zone and stripe architecture (zebrin I—Leclerc et al., 1988; HSP25—Armstrong et al., 2001). Similarly, cerebellar anlagen dissected from embryos at E12–E15 (prior to any contact with afferents—Paradies and Eisenman, 1993; Grishkat and Eisenman, 1995) and transplanted into either the anterior chamber of the eye or the neocortex of adult hosts (Wassef et al., 1990) had zebrin II+/− Purkinje cells in the mature grafts. Furthermore, zebrin+ Purkinje cells are more numerous in cultures of posterior cerebellum than anterior cerebellum, consistent with the expression pattern seen in vivo (Leclerc et al., 1988; Hawkes, unpublished data). Finally, several experiments have suggested a correlation between the time when a Purkinje cell is born and its final mediolateral position in the mature cerebellum (Hashimoto and Mikoshiba, 2003; Larouche et al., 2006) suggesting that Purkinje cells' adult phenotypes are specified shortly after their birth in the VZ.
What is known of Purkinje cell subtype specification? EBF2 is one of four members of a family of helix-loop-helix transcription factors highly conserved in evolution (reviewed in Dubois and Vincent, 2001; Liberg et al., 2002) that couple cell cycle exit to the onset of neuronal differentiation and migration (Garcia-Dominguez et al., 2003). Three of these (Ebf1-Ebf3) encode transcriptional activators expressed in cerebellar development (Figure 2, Croci et al., 2006). While no cerebellar defects have been described in Ebf1 or Ebf3 mutants, Ebf2 null mice feature a small cerebellum and apoptotic cell death of migrating and post-migratory Purkinje neurons. Of the Purkinje cells that survive, a major fraction is transdifferentiated into the zebrin II+ phenotype (Croci et al., 2006). An analysis of molecular markers of Purkinje cell subtypes revealed that EBF2 acts specifically to repress the zebrin II+ subtype, rather than to maintain the zebrin II– one (Chung et al., 2008). Interestingly, unlike EBF3, EBF2 is sensitive to Notch-mediated repression in Xenopus neurulae (Pozzoli et al., 2001), both at the transcriptional and at the functional level, suggesting that Notch signaling may affect the determination of Purkinje cell subtypes by modulating EBF2, for instance by switching off Ebf2 expression in the early born Purkinje cell population. Consistent with this hypothesis, genetic tagging of EBF2+ cells by using an Ebf2-Cre transgene reveals that all Purkinje cells are initially Ebf2+ (Consalez, unpublished). Conditional overexpression experiments are now required to elucidate the crucial stages at which EBF2 affects Purkinje cell subtype specification in pre- and postnatal development.
The Migration of Purkinje Cells to Form Embryonic Clusters
Post-mitotic Purkinje cells migrate from the VZ and stack in a layer with the earliest-born located dorsally (and becoming zebrin II+) and the youngest ventrally (and becoming EBF2+: Figure 2). Subsequently the layer undergoes a quite complicated reorganization (Miyata et al., 2010), possibly involving cell-signaling molecules including cadherins (e.g., Neudert and Redies, 2008; Redies et al., 2011) and ephrins (e.g., Karam et al., 2000; Sentürk et al., 2011), to yield a stereotyped array of embryonic Purkinje cell clusters with multiple molecular phenotypes. Grafts of dissociated Purkinje cells also organize into discrete zebrin+/− compartments, pointing to cell-cell adhesion molecules as possible organizers (Rouse and Sotelo, 1990). Each Purkinje cell cluster is separated from its neighbors by narrow gaps (“raphes”), later filled by migrating granule cells. In general terms, expression data show up to 10 embryonic clusters, arrayed symmetrically from medial to lateral on each side of the midline.
Cluster Architecture in the Embryonic Cerebellum
The reorganization of the early lamina results in a highly reproducible array of Purkinje cell clusters that can be distinguished through the differential expression of numerous molecules. The expression profiles are of three kinds. First, there are molecules that are selectively expressed at some time during embryogenesis but subsequently disappear and are not expressed in the adult (e.g., neurogranin—Larouche et al., 2006). Secondly, some molecules are selectively expressed during embryogenesis but are expressed by all Purkinje cells in the adult (e.g., calbindin—Wassef et al., 1985). Thirdly, a few molecules are selectively expressed by Purkinje cell subsets both in the embryo and the adult (e.g., PLCβ4—Marzban et al., 2007); and finally, in some cases expression reveals one pattern in the neonate and a different one in the adult (e.g., HSP25—Armstrong et al., 2001).
Examples of embryonic cluster markers include:
Calbindin (Calb1) is a major calcium binding protein that acts as a buffer to protect neurons from neurotoxicity. At around P0, calbindin expression defines three Purkinje cell clusters on each side of the midline (Wassef et al., 1985; Larouche et al., 2006). In the adult cerebellum, calbindin is expressed uniformly by all Purkinje cells
Phospholipase Cβ4 (PLCβ4) is a signal transducer. PLCβ4-immunoreactive Purkinje cells in neonatal mice reveal two, three, and four parasagittal domains in the AZ, CZ, and PZ respectively. Later, differential expression of PLCβ4 in the AZ and PZ reveal a striped pattern in the mature cerebellar cortex (Marzban et al., 2007).
Engrailed-2 (En2) is a transcription factor important for the differentiation of Purkinje cells and the adult (“late-onset”) banding pattern. En2 expression, labels three distinct cluster domains at E17.5 but expression is suppressed in Purkinje cells after birth (Millen et al., 1995).
Cadherins mediate cell adhesion and play fundamental roles in the growth and development of many cells. Purkinje cell clusters express multiple members of this superfamily and some, such as cdh8, pch7, and pcdh10, are expressed differentially. For example, in the mouse cerebellar cortex at P3 cdh8-immunoreactive Purkinje cells form two parasagittal clusters each side of the midline, there are three pch7+ clusters, and a single pcdh10 cluster. In some cases, such as pcdh10, expression is maintained and the cerebellum displays a striped pattern in the adult cortex (Redies et al., 2011).
Heat shock protein 25 (HSP25) is involved in stress resistance by acting as a chaperone that binds to and stabilizes the active conformations of other proteins. At P1, the anterior lobe of the mouse cerebellum presents two distinct pairs of clusters of HSP25+ Purkinje cells arrayed symmetrically about the midline in the AZ and PZ (Armstrong et al., 2001). During later postnatal development HSP25 is transiently expressed by all Purkinje cells, until in the adult expression becomes restricted to a quite different pattern of stripes (in the CZ and NZ: Armstrong et al., 2000, 2001).
Purkinje cell protein 2-lacZ transgene (L7/pcp2-lacZ): pcp2 is a G-protein regulator that is widely expressed in Purkinje cells. Around P0, the differential expression pattern of an L7/pcp2-lacZ transgene reveals three distinct compartments in each hemicerebellum (Oberdick et al., 1993; Ozol et al., 1999). In the adult cerebellum, L7/pcp2-lacZ expression remains in stripes of Purkinje cells, especially in the AZ and PZ (Ozol et al., 1999).
Synaptotagmin IV (Syt IV) is involved in early neural differentiation including axonal growth and the formation and consolidation of synapses. At P0, Syt IV is weakly expressed in select Purkinje cell clusters. From P15 onward, all Purkinje cells are Syt IV+ (Berton et al., 1997).
Neurogranin (Nrgn) is a neural calmodulin-binding protein thought to play an important role in synaptic transmission and neuronal plasticity. At E17, Nrgn expression in the AZ and PZ reveals three and four parasagittal pairs of neurogranin expressing Purkinje cell clusters respectively (Larouche et al., 2006). These disappear in the adult.
Inositol 1,4,5-trisphosphate (IP3) receptor-lacZ transgene (IP3Rnls-lacZ): IP3R is a ligand-gated calcium channel, which is highly expressed in Purkinje cells. From E15 to P0, two clusters of Purkinje cells are selectively labeled on either side of the midline. Transgene expression continues to reveal heterogeneous Purkinje cells stripes in both the vermis and the hemisphere in the adult (Furutama et al., 2010).
Wnt7b is a signaling molecule involved in CNS development. At E18, Wnt7b expression reveals three mediolateral Purkinje cells clusters: Wnt7b expression is shut down in the adult (Hashimoto and Mikoshiba, 2003).
Olfactory marker protein-lacZ transgene (OMP-lacZ): The pattern of expression of an OMP-lacZ fusion gene (from E14.5 to P0) demonstrates three clusters on each side of the cerebellar midline. In the adult cerebellum, the pattern of transgene expression continues to reveal a striped pattern, restricted to the posterior lobe (Nunzi et al., 1999).
Early B-cell factor 2 (EBF2): Sections through the mouse cerebellum show EBF2 is expressed shortly after birth in multiple stripes and wholemount staining of adult cerebellum shows EBF2-lacZ is expressed in stripes restricted to the AZ and CZ, equivalent to the distribution of the zebrin II– Purkinje cell subset (Croci et al., 2006).
Cyclic GMP-dependent protein kinase (cGK) is implicated in multiple biological functions, including axon guidance, synaptic plasticity and learning. Transverse sections of rat cerebellum taken between E17–P3 reveal two discrete clusters of Purkinje cells that are immunoreactive for cGK. In adults, all Purkinje cell express cGK (De Camilli et al., 1984; Wassef et al., 1985).
Ephrin type-A receptor 4 (EphA4): is a tyrosine kinase receptor. Transverse sections through the mouse cerebellum at E18.5 display four distinct EphA4+ clusters of Purkinje cells (Hashimoto and Mikoshiba, 2003). In the adult cerebellum, EphA4 expression appears homogenous, except perhaps for some areas of the hemispheres (Karam et al., 2000).
PEP-19: is a developmentally regulated polypeptide that modulates calmodulin function. The expression pattern demonstrates three clusters on each side of the cerebellar midline (Herrup and Kuemerle, 1997). PEP19 expressed in all Purkinje cells of the adult rat cerebellum (Mugnaini et al., 1987).
While these, and other, markers reveal embryonic cerebellar complexity, the relationships between the various topographic maps are poorly understood. A speculative but most useful synthesis is presented in Herrup and Kuemerle (1997).
From Clusters to Stripes
Purkinje cell cluster dispersal is triggered at around birth by Reelin secreted by the external granular layer (D'Arcangelo et al., 1995, 1997; Miyata et al., 1997; Tissir and Goffinet, 2003: Figure 2). Reelin binds two receptors on Purkinje cells—Apolipoprotein E receptor 2 (Apoer2) and the very low density lipoprotein receptor (Vldlr: Trommsdorff et al., 1999; Hiesberger et al., 1999). Binding induces receptor clustering (Strasser et al., 2004) and activates a protein kinase cascade leading to tyrosine phosphorylation of the docking protein Disabled (Dab1: Goldowitz et al., 1997; Howell et al., 1997; Sheldon et al., 1997; Gallagher et al., 1998; Rice et al., 1998). Downstream of Dab1 are multiple kinase pathways Src and Fyn tyrosine kinases (Bock and Herz, 2003; Kuo et al., 2005), cyclin-dependant kinase 5 (Ohshima and Mikoshiba, 2002 etc.). The end result is thought to be a drop in mutual Purkinje cell-Purkinje cell adhesion, thereby freeing the embryonic clusters to disperse into stripes.
When this pathway is disrupted by mutation of Reelin (reeler: D'Arcangelo et al., 1995, 1997), Reelin receptors (Apoer2/Vldlr double null: Trommsdorff et al., 1999) or the Dab1 docking protein (Dab1–/–: Howell et al., 1997) all cluster dispersal is blocked. However, in contrast to the full reeler phenotype with no embryonic cluster dispersal, several mutations cause a partial reeler phenotype—some Purkinje cells remain as ectopic clusters in the cerebellar core while most disperse normally to form stripes (weaver—Armstrong and Hawkes, 2001; rostral cerebellar malformation—Ackerman et al., 1997; cerebellar deficient folia—Beirebach et al., 2001, etc.). For some of these, mutations in the human homologs are known similar cerebellar phenotypes (e.g., Reelin—Hong et al., 2000; Vldlr—Boycott et al., 2005).
In the embryo the cluster is ∼10 Purkinje cells deep. As the clusters disperse into adult stripes the Purkinje cells spread to form a monolayer. Because dispersal occurs primarily in the anteroposterior plane, as the lobules of the cerebellum form, the rostrocaudal length of the cerebellum increases ∼25-fold while the width of the vermis increases only ∼1.5-fold (Gallagher et al., 1998). As a result the clusters string out into long parasagittal stripes. Most adult stripe markers are first expressed during this period. A few already show more-or-less adult patterns of restriction by around P5 (e.g., PLCβ4—Marzban et al., 2007) but most—including zebrin II (Lannoo et al., 1991a,b)—are first expressed at around this time but go through a “global expression” phase in which they are expressed by all Purkinje cells (e.g., HSP25—Armstrong et al., 2001; zebrin II—Lannoo et al., 1991a; Rivkin and Herrup, 2003; OMP-lacZ—Nunzi et al., 1999) before they are selectively down-regulated and the stripe architecture matures by P20.
What is the topographical relationship between the embryonic clusters and the adult stripes? By E18, numerous Purkinje cell molecular markers show restriction to subsets of clusters. The accumulated data from expression mapping of single markers suggest the possibility of a straightforward embryonic architecture: all known early markers appear to be restricted to the same schema with no more than ∼10 clusters on each side of the midline. So why are there many more adult Purkinje cell stripes than there are embryonic clusters (several hundred stripes vs. a few dozen clusters)? One explanation is that clusters are much more complex than is generally appreciated. By this view, the elaborate adult topography arises because each “simple” embryonic cluster in fact comprises multiple sub-clusters. In some cases there may be internal partitions (e.g., a medial vs. a lateral component of a cluster, each becoming a separate stripe in the adult); in other cases, Purkinje cells of different phenotypes may be intermingled within a cluster but segregate into separate stripes as the cluster transforms into stripes. Alternatively, each embryonic cluster may be homogeneous and additional complexity introduced into the adult map because individual clusters disperse into multiple stripes of the same adult phenotype. One previous study supports “complex dispersal”—in the weaver mouse two clusters fail to disperse and three adult stripes are missing, all of the zebrin II+/HSP25+ phenotype (Armstrong and Hawkes, 2001). Sillitoe et al. (2009) reveal a similar story by using a pcp2-CreER-IRES-hAP transgene to tag three bilateral clusters on approximately E15 and show they yielded zebrin II+ Purkinje cells of nine adult stripes. On the other hand, in some cases several embryonic clusters merge to form a single stripe. The clearest example are the zebrin II–/PLCβ4+ stripes in the vermis of the AZ, which are seen to be subdivided into triplets by the pattern of mossy fiber innervation (Ji and Hawkes, 1994) and arise from the fusion of three perinatal PLCβ4+ clusters (Marzban et al., 2007).
Role of Programmed Purkinje Cell Death?
Finally, during the perinatal period it is clear that significant Purkinje cell death occurs (reviewed in Vogel, 2002: Figure 2). Does this play a role in the sculpting of cerebellar topography? Two complementary hypotheses can be considered. First, studies of naturally occurring cell death in the cerebellum have identified a spatial organization to Purkinje cell apoptosis (“hot spots”: Jankowski et al., 2009) that correlates with stripe boundaries in the adult, and propose the interesting hypothesis that cell death may sharpen the acellular raphes between clusters. In addition, naturally occurring cell death could be an error-correction mechanism. A striking feature of adult cerebellar topography is its high reproducibility between individuals and its attendant low error rate (e.g., zebrin II+ Purkinje cells are very rarely seen in zebrin II– stripes). If stripes derive from clusters, and stripes have no errors, then either clusters have no errors (and migration from the VZ to the clusters is perfect) or errors that occur during cluster formation are subsequently eliminated. In this context it is interesting that many Purkinje cells—perhaps as many as a third—undergo cell death by apoptosis during the perinatal period (Dusart et al., 2006; Jankowski et al., 2009). This suggests the hypothesis that perinatal apoptosis might eliminate Purkinje cells that wind up in the wrong embryonic cluster (possibly via a local insulin-like growth factor 1 pathway—Croci et al., 2011; see also Jung et al., 2008). Purkinje cell ectopia is not lethal per se: for example, clusters that fail to disperse normally do not die [e.g., reeler (Goffinet, 1983; Edwards et al., 1994), Vldlr–/–:Apoer2–/– (Larouche et al., 2008), Dab1–/– (Howell et al., 1997)], and Purkinje cells located ectopically in the molecular or granular layers survive indefinitely (e.g., Rouse and Sotelo, 1990; Carletti et al., 2008). Rather, one might evoke a community effect (à la Yang et al., 2002), such that being in the wrong cluster during development leads to apoptosis.
Purkinje Cell Architecture as a Scaffold for Cerebellar Topography
It is generally believed that the Purkinje cell architecture is the scaffolding around which many other cerebellar components are organized (e.g., reviewed in Sotelo and Wassef, 1991). For example, both climbing fiber and mossy fiber afferents terminate in the cerebellum as stripes that align with those revealed by stripe antigens (e.g., climbing fibers—Chédotal et al., 1997; Sotelo and Chédotal, 2005; mossy fibers—Sotelo and Wassef, 1991; Ji and Hawkes, 1995; Armstrong et al., 2009). In some cases, this can be very precise: for example somatostatin-immunoreactive mossy fibers terminate precisely beneath a very small subset of Purkinje cell stripes (∼2%) that constitutively express HSP25 (Armstrong et al., 2009). The alignment of the afferent and Purkinje cell (= efferent) maps is established early in cerebellar development where the earliest mossy fiber topography is seen as transient, possibly functional, contacts between mossy fibers and Purkinje cells (e.g., Mason and Gregory, 1984; Takeda and Maekawa, 1989) in specific embryonic clusters (Grishkat and Eisenman, 1995; Paradies et al., 1996). When the embryonic clusters disperse into stripes the afferents appear to move with them, thereby retaining the topographic relationship with a particular Purkinje cell subset. During postnatal development, mossy fibers move to the granular layer but stay aligned with the Purkinje cell stripe (e.g., Arsénio Nunes and Sotelo, 1985; Ji and Hawkes, 1995). A similar mechanism seems to serve to guide cerebellar interneurons to their specific stripe locations (unipolar brush cells—Chung et al., 2009a,b; Golgi cells—Sillitoe et al., 2008) and boundaries between stripes restrict the mediolateral spread of Golgi cell dendritic arbors (Sillitoe et al., 2008).
Finally, a different, and not well-understood, process also restricts granule cell dispersal. There are several subclasses of granule cell based both on gene expression (e.g., reviewed in Hawkes and Eisenman, 1997; Ozol and Hawkes, 1997) and lineage (Hawkes et al., 1998). Transverse boundaries that separate granule cell lineages align with transverse zone boundaries identified in the Purkinje cell scaffold. How this comes about is not understood.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
These studies were supported by the Canadian Institutes of Health Research (R. Hawkes). G.G. Consalez was supported by Ataxia UK and the Compagnia di San Paolo, Torino, Italy.
References
Ackerman, S. L., Kozak, L. P., Przyborski, S. A., Rund, L. A., Boyer, B. B., and Knowles, B. B. (1997). The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature 386, 838–842.
Ahn, A. H., Dziennis, S., Hawkes, R., and Herrup, K. (1994). The cloning of zebrin II reveals its identity with aldolase C. Development 120, 2081–2090.
Apps, R., and Garwicz, M. (2005). Anatomical and physiological foundations of cerebellar information processing. Nat. Rev. Neurosci. 6, 297–311.
Apps, R., and Hawkes, R. (2009). Cerebellar cortical organization: a one-map hypothesis. Nat. Rev. Neurosci. 10, 670–681.
Armstrong, C., Chung, S-H., Armstrong, J., Hochgeschwender, U., Jeong, Y-G., and Hawkes, R. (2009). A novel somatostatin-immunoreactive mossy fiber pathway associated with HSP25-immunoreactive Purkinje cell stripes in the mouse cerebellum. J. Comp. Neurol. 517, 524–538.
Armstrong, C. L., and Hawkes, R. (2001). Selective failure of Purkinje cell dispersion in the cerebellum of the weaver mouse. J. Comp. Neurol. 439, 151–161.
Armstrong, C. L., Krueger-Naug, A. M., Currie, W. C., and Hawkes, R. (2000). Constitutive expression of the 25kDa heat shock protein Hsp25 reveals novel parasagittal stripes of Purkinje cells in the adult mouse cerebellar cortex. J. Comp. Neurol. 416, 383–397.
Armstrong, C. L., Krueger-Naug, A. M. R., Currie, R. W., and Hawkes, R. (2001). Expression of heat-shock protein Hsp25 in mouse Purkinje cells during development reveals novel features of cerebellar compartmentation. J. Comp. Neurol. 429, 7–21.
Arsénio Nunes, M. L., and Sotelo, C. (1985). Development of the spinocerebellar system in the postnatal rat. J. Comp. Neurol. 237, 291–306.
Bally-Cuif, L., Alvarado-Mallart, R. M., Darnell, D. K., and Wassef, M. (1992). Relationship between Wnt-1 and En-2 expression domains during early development of normal and ectopic met-mesencephalon. Development 115, 999–1009.
Bally-Cuif, L., Dubois, L., and Vincent, A. (1998). Molecular cloning of Zcoe2, the zebrafish homolog of Xenopus Xcoe2 and mouse EBF-2, and its expression during primary neurogenesis. Mech. Dev. 77, 85–90.
Battiste, J., Helms, A. W., Kim, E. J., Savage, T. K., Lagace, D. C., Mandyam, C. D., Eisch, A. J., Miyoshi, G., and Johnson, J. E. (2007). Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development 134, 285–293.
Beirebach, E., Park, C., Ackerman, S. L., Goldowitz, D., and Hawkes, R. (2001). Abnormal dispersion of a Purkinje cell subset in the mouse mutant cerebellum deficient folia (cdf). J. Comp. Neurol. 436, 42–51.
Berton, F., Iborra, C., Boudier, J. A., Seagar, M. J., and Marquèze, B. (1997). Developmental regulation of synaptotagmin I, II, III, and IV mRNAs in the rat CNS. J. Neurosci. 17, 1206–1216.
Bock, H. H., and Herz, J. (2003). Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13, 18–26.
Boycott, K. M., Flavelle, S., Bureau, A., Glass, H. C., Fujiwara, T. M., Wirrell, E., Davey, K., Chudley, A. E., Scott, J. N., McLeod, D. R., and Parboosingh, J. S. (2005). Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive hypoplasia with cerebral gyral simplification. Am. J. Hum. Genet. 77, 477–483.
Brochu, G., Maler, L., and Hawkes, R. (1990). Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J. Comp. Neurol. 291, 538–552.
Carletti, B., Williams, I. M., Nakajima, K., Magrassi, L., and Rossi, F. (2008). Time constrainsts and positional cues in the developing cerebellum regulate Purkinje cell placement in the cortical architecture. Dev. Biol. 317, 147–160.
Chédotal, A., Bloch-Gallego, E., and Sotelo, C. (1997). The embryonic cerebellum contains topographic cues that guide developing inferior olivary axons. Development 124, 861–870.
Chen, G., Hanson, C. L., and Ebner, T. J. (1996). Functional parasagittal compartments in the rat cerebellar cortex: an in vivo optical imaging study using neutral red. J. Neurophysiol. 76, 4169–4174.
Chizhikov, V. V., Lindgren, A. G., Currle, D. S., Rose, M. F., Monuki, E. S., and Millen, K. J. (2006). The roof plate regulates cerebellar celltype specification and proliferation. Development 133, 2793–2804.
Chizhikov, V., and Millen, K. J. (2003). Development and malformations of the cerebellum in mice. Mol. Genet. Metab. 80, 54–65.
Chockkan, V., and Hawkes, R. (1994). Functional and antigenic maps in the rat cerebellum: zebrin compartmentation and vibrissal receptive fields in lobule IXa. J. Comp. Neurol. 345, 33–45.
Chung, S-H., Marzban, H., Croci, L., Consalez, G. G., and Hawkes, R. (2008). Purkinje cell subtype specification in the cerebellar cortex: EBF2 acts to repress the zebrin II–negative Purkinje cell phenotype. Neuroscience 153, 721–732.
Chung, S-H., Marzban, H., and Hawkes, R. (2009a). Compartmentation of the cerebellar nuclei of the mouse. Neuroscience 161, 123–138.
Chung, S-H., Sillitoe, R. V., Croci, L., Baldoni, A., Consalez, G., and Hawkes, R. (2009b). Unipolar brush cells use Purkinje cells to restrict their topography. Neuroscience 164, 1496–1508.
Croci, L., Barili, V., Chia, D., Massimino, L., van Vugt, R., Masserdotti, G., Longhi, R., Rotwein, P., and Consalez, G. G. (2011). Local insulin-like growth factor I expression is essential for Purkinje neuron survival at birth. Cell Death Differ. 18, 48–59.
Croci, L., Chung, S-H., Masserdotti, G., Gianola, S., Motti, E., Tonini, R., Braida, D., Bizzoca, A., Gennarini, G., Corradi, A., Sala, M., Rossi, F., Hawkes, R., and Consalez, G. G. (2006). A key role for the HLH transcription factor EBF2 (COE2, O/E-3) in Purkinje neuron migration and cerebellar cortical topography. Development 133, 2719–2729.
Crossley, P. H., Martinez, S., and Martin, G. (1996). Midbrain development induced by FGF8 in the chick embryo. Nature 380, 66–68.
Dalgard, C. L., Zhou, Q., Lundell, T. G., and Doughty, M. L. (2011). Altered gene expression in the emerging cerebellar primordium of Neurog1−/− mice. Brain Res. 1388, 12–21.
D'Arcangelo, G., Miao, G. G., Chen, S. C., Soares, H. D., Morgan, J. I., and Curran, T. (1995). A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723.
D'Arcangelo, G., Nakajima, K., Miyata, T., Ogawa, M., Mikoshiba, K., and Curran, T. (1997). Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 17, 23–31.
De Camilli, P., Miller, P. E., Levitt, P., Walter, U., and Greengard, P. (1984). Anatomy of cerebellar Purkinje cells in the rat determined by a specific immunohistochemical marker. Neuroscience 11, 761–817.
Demilly, A., Reeber, S. L., Gebre, S. A., and Sillitoe, R. V. (2011). Neurofilament heavy chain expression reveals a unique parasagittal stripe topography in the mouse cerebellum. Cerebellum 10, 409–421.
Dubois, L., and Vincent, A. (2001). The COE-Collier/Olf1/EBF transcription factors: structural conservation and diversity of developmental functions. Mech. Dev. 108, 3–12.
Dusart, I., Guenet, J. L., and Sotelo, C. (2006). Purkinje cell death: differences between developmental cell death and neurodegenerative death in mutant mice. Cerebellum 5, 163–173.
Edwards, M. A., Leclerc, N., Crandall, J. E., and Yamamoto, M. (1994). Purkinje cell compartments in the reeler mutant mouse as revealed by Zebrin II and 9-0-acetylated glycolipid antigen expression. Anat. Embryol. (Berl.) 190, 417–428.
Eisenman, L. M. (2000). Antero-posterior boundaries and compartments in the cerebellum: evidence from selected neurological mutants. Prog. Brain Res. 124, 23–30.
Eisenman, L. M., and Hawkes, R. (1993). Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography. J. Comp. Neurol. 335, 586–605.
Furutama, D., Morita, N., Takano, R., Sekine, Y., Sadakata, T., Shinoda, Y., Hyashi, K., Mishima, Y., Mikoshiba, K., Hawkes, R., and Furuishu, T. (2010). Expression of the IP3R1 promoter-driven nls-lacZ transgene in Purkinje cell parasagittal arrays of developing mouse cerebellum. J. Neurosci. Res. 88, 2810–2825.
Gallagher, E., Howell, B. W., Soriano, P., Cooper, J. A., and Hawkes, R. (1998). Cerebellar abnormalities in the disabled (mdab1-1) mouse. J. Comp. Neurol. 402, 238–251.
Garcia-Dominguez, M., Poquet, C., Garel, S., and Charnay, P. (2003). Ebf gene function is required for coupling neuronal differentiation and cell cycle exit. Development 130, 6013–6025.
Goffinet, A. M. (1983). The embryonic development of the cerebellum in normal and reeler mutant mice. Anat. Embryol. 168, 73–86.
Goldowitz, D., Cushing, R. C., Laywell, E., D'Arcangelo, G., Sheldon, M., Sweet, H. O., Davisson, M., Steindler, D., and Curran, T. (1997). Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of Reelin. J. Neurosci. 17, 8767–8777.
Grimaldi, P., Parras, C., Guillemot, F., Rossi, F., and Wassef, M. (2009). Origins and control of the differentiation of inhibitory interneurons and glia in the cerebellum. Dev. Biol. 328, 422–433.
Grishkat, H. L., and Eisenman, L. M. (1995). Development of the spinocerebellar projection in the prenatal mouse. J. Comp. Neurol. 363, 93–108.
Hallem, J. S., Thompson, J., Gundappa-Sulur, S., Hawkes, R., Bjallie, J. G., and Bower, J. M. (1999). Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIa of the rat. Neuroscience 93, 1083–1094.
Hashimoto, M., and Mikoshiba, K. (2003). Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. J. Neurosci. 23, 11342–11351.
Hawkes, R., Colonnier, M., and Leclerc, N. (1985). Monoclonal antibodies reveal sagittal banding in the rodent cerebellar cortex. Brain Res. 333, 359–365.
Hawkes, R., and Eisenman, L. M. (1997). Stripes and zones: the origins of regionalization of the adult cerebellum. Perspect. Dev. Neurobiol. 5, 95–104.
Hawkes, R., Faulkner-Jones, B., Tam, P., and Tan, S. S. (1998). Pattern formation in the cerebellum of murine embryonic stem cell chimeras. Eur. J. Neurosci. 10, 790–793.
Hawkes, R., and Leclerc, N. (1986). Immunocytochemical demonstration of topographic ordering of Purkinje cell axon terminals in the fastigial nuclei of the rat. J. Comp. Neurol. 244, 481–491.
Hawkes, R., and Turner, R. W. (1994). Compartmentation of NADPH-diaphorase activity in the mouse cerebellar cortex. J. Comp. Neurol. 346, 499–516.
Helms, A. W., Battiste, J., Henke, R. M., Nakada, Y., Simplicio, N., Guillemot, F., and Johnson, J. E. (2005). Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development 132, 2709–2719.
Henke, R. M., Savage, T. K., Meredith, D. M., Glasgow, S. M., Hori, K., Dumas, J., MacDonald, R. J., and Johnson, J. E. (2009). Neurog2 is a direct downstream target of the Ptf1a-Rbpj transcription complex in dorsal spinal cord. Development 136, 2945–2954.
Herrup, K., and Kuemerle, B. (1997). The compartmentalization of the cerebellum. Annu. Rev. Neurosci. 20, 61–90.
Hiesberger, T., Trommsdorff, M., Howell, B. W., Goffinet, A., Mumby, M. C., Cooper, J. A., and Herz, J. (1999). Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24, 481–489.
Hong, S. E., Shugart, Y. Y., Huang, D. T., Shahwan, S. A., Grant, P. E., Hourihane, J. O., Martin, N. D., and Walsh, C. A. (2000). Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 26, 93–96.
Hoshino, M. (2006). Molecular machinery governing GABAergic neuron specification in the cerebellum. Cerebellum 5, 193–198.
Hoshino, M., Nakamura, S., Mori, K., Kawauchi, T., Terao, M., Nishimura, Y. V., Fukuda, A., Fuse, T., Matsuo, N., Sone, M., Watanabe, M., Bito, H., Terashima, T., Wright, C. V., Kawaguchi, Y., Nakao, K., and Nabeshima, Y. (2005). Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron 47, 201–213.
Howell, B. W., Hawkes, R., Soriano, P., and Cooper, J. A. (1997). Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 389, 733–777.
Jankowski, J., Miething, A., Schilling, K., and Baader, S. L. (2009). Physiological Purkinje cell death is spatiotemporally organized in the developing mouse cerebellum. Cerebellum 8, 277–290.
Ji, Z., and Hawkes, R. (1994). Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience 61, 935–954.
Ji, Z., and Hawkes, R. (1995). Developing mossy fibers terminal fields in the rat cerebellar cortex may segregate because of Purkinje cell compartmentation and not competition. J. Comp. Neurol. 359, 197–212.
Joyner, A. L. (1996). Engrailed, Wnt and Pax genes regulate midbrain-hindbrain development. Trends Genet. 12, 15–20.
Jung, A. R., Kim, T. W., Rhyu, I. J., Kim, H., Lee, Y. D., Vinsant, S., Oppenheim, R. W., and Sun, W. (2008). Misplacement of Purkinje cells during postnatal development in Bax knock-out mice: a novel role for programmed cell death in the nervous system? J. Neurosci. 28, 2941–2948.
Karam, S. D., Burrows, R. C., Logan, C., Koblar, S., Pasquale, E. B., and Bothwell, M. (2000). Eph receptors and ephrins in the developing chick cerebellum: relationship to sagittal patterning and granule cell migration. J. Neurosci. 20, 6488–6500.
Kim, E. J., Hori, K., Wyckoff, A., Dickel, L. K., Koundakjian, E. J., Goodrich, L. V., and Johnson, J. E. (2011). Spatiotemporal fate map of neurogenin1 (Neurog1) lineages in the mouse central nervous system. J. Comp. Neurol. 519, 1355–1370.
Kuo, G., Arnaud, L., Kronstad-O'Brien, P., and Cooper, J. A. (2005). Absence of Fyn and Src causes a reeler-like phenotype. J. Neurosci. 25, 8578–8586.
Lannoo, M. J., Brochu, G., Maler, L., and Hawkes, R. (1991a). Zebrin II immunoreactivity in the rat and in the weakly electric teleost Eigenmannia (Gymnotiformes) reveals three modes of Purkinje cell development. J. Comp. Neurol. 310, 215–233.
Lannoo, M., Ross, L., Maler, L., and Hawkes, R. (1991b). Development of the cerebellum and its extracerebellar Purkinje-cell projection in teleost fishes as determined by zebrin-II immunocytochemistry. Prog. Neurobiol. 37, 329–363.
Larouche, M., Beffert, U., Herz, J., and Hawkes, R. (2008). The Reelin receptors Apoer2 and Vldlr coordinate the patterning of Purkinje cell topography in the developing mouse cerebellum. PLoS One 3:e1653. doi: 10.1371/journal.pone.0001653
Larouche, M., Che, P. M., and Hawkes, R. (2006). Neurogranin expression identifies a novel array of Purkinje cell parasagittal stripes during mouse cerebellar development. J. Comp. Neurol. 494, 215–227.
Larouche, M., and Hawkes, R. (2006). From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum 5, 77–88.
Leclerc, N., Gravel, C., and Hawkes, R. (1988). Development of parasagittal zonation in the rat cerebellar cortex: mabQ113 antigenic bands are created postnatally by the suppression of antigen expression in a subset of Purkinje cells. J. Comp. Neurol. 273, 399–420.
Leclerc, N., Schwarting, G., Herrup, K., Hawkes, R., and Yamamoto, M. (1992). Compartmentation in mammalian cerebellum: zebrin II and P-path antibodies define three classes of sagittally organized bands of Purkinje cells. Proc. Natl. Acad. Sci. U.S.A. 89, 5006–5010.
Liberg, D., Sigvardsson, M., and Akerblad, P. (2002). The EBF/Olf/Collier family of transcription factors: regulators of differentiation in cells originating from all three embryonal germ layers. Mol. Cell. Biol. 22, 8389–8397.
Liu, A., Losos, K., and Joyner, A. L. (1999). FGF8 can activate Gbx2 and transform regions of the rostral mouse into a hindbrain fate. Development 126, 4827–4838.
Lundell, T. G., Zhou, Q., and Doughty, M. L. (2009). Neurogenin1 expression in cell lineages of the cerebellar cortex in embryonic and postnatal mice. Dev. Dyn. 238, 3310–3325.
Malgaretti, N., Pozzoli, O., Bosetti, A., Corradi, A., Ciarmatori, S., Panigada, M., Bianchi, M. E., Martinez, S., and Consalez, G. G. (1997). Mmot1, a new helix-loop-helix transcription factor gene displaying a sharp expression boundary in the embryonic mouse brain. J. Biol. Chem. 272, 17632–17639.
Martinez, S., Crossley, P. H., Cobos, I., Rubenstein, J. L. R., and Martin, G. R. (1999). FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via repressive effect on Otx2 expression. Development 126, 1189–1200.
Marzban, H., Chung, S-H., Kheradpezhouh, M., Watanabe, M., Voogd, J., and Hawkes, R. (2010). Antigenic compartmentation of the cerebellar cortex in the chicken (Gallus domesticus). J. Comp. Neurol. 518, 2221–2239.
Marzban, H., Chung, S-H., Watanabe, M., and Hawkes, R. (2007). Phospholipase Cß4 expression reveals the continuity of cerebellar topography through development. J. Comp. Neurol. 502, 857–871.
Marzban, H., and Hawkes, R. (2011). On the architecture of the posterior zone of the cerebellum. Cerebellum 10, 422–434.
Marzban, H., Kim, C-T., Doorn, D., Chung, S-H., and Hawkes, R. (2008). A novel transverse expression domain in the mouse cerebellum revealed by a neurofilament-associated antigen. Neuroscience 153, 1190–1201.
Marzban, H., Sillitoe, R. V., Hoy, M., Chung, S-H., Rafuse, V. F., and Hawkes, R. (2004). Abnormal HNK-1 expression in the cerebellum of an N-CAM null mouse. J. Neurocytol. 33, 117–130.
Mason, C. A., and Gregory, E. (1984). Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. J. Neurosci. 4, 1715–1735.
McMahon, A. P., Joyner, A. L., Bradley, A., and McMahon, J. A. (1992). The midbrain-hindbrain? phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcitum. Cell 69, 581–595.
Meek, J., Hafmans, T. G. M., Maler, L., and Hawkes, R. (1992). Distribution of zebrin-II in the gigantocerebellum of the mormyrid fish Gnathonemus petersii compared with other teleosts. J. Comp. Neurol. 316, 17–31.
Miale, I., and Sidman, R. L. (1961). An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp. Neurol. 4, 277–296.
Millen, K. J., Hui, C. C., and Joyner, A. L. (1995). A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development 121, 3935–3945.
Minaki, Y., Nakatani, T., Mizuhara, E., Inoue, T., and Ono, Y. (2008). Identification of a novel transcriptional corepressor, Corl2, as a cerebellar Purkinje cell-selective marker. Gene Expr. Patterns 8, 418–423.
Miyata, T., Nakajima, K., Mikoshiba, K., and Ogawa, M. (1997). Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody. J. Neurosci. 17, 3599–3609.
Miyata, T., Ono, Y., Okamoto, M., Masaoka, M., Sakakibara, A., Kawaguchi, A., Mitsuhiro, M., and Ogawa, M. (2010). Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum. Neural Dev. 5, 23.
Mizuhara, E., Minaki, Y., Nakatani, T., Kumai, M., Inoue, T., Muguruma, K., Sasai, Y., and Ono, Y. (2010). Purkinje cells originate from cerebellar ventricular zone progenitors positive for Neph3 and E-cadherin. Dev. Biol. 338, 202–214.
Morales, D., and Hatten, M. E. (2006). Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J. Neurosci. 26, 12226–12236.
Mugnaini, E., Berrebi, A. S., Dahl, A. L., and Morgan, J. I. (1987). The polypeptide PEP-19 is a marker for Purkinje neurons in cerebellar cortex and cartwheel neurons in the dorsal cochlear nucleus. Arch. Ital. Biol. 126, 41–67.
Nakamura, H., Sato, T., and Suzuki-Hirano, A. (2008). Isthmus organizer for mesencephalon and metencephalon. Dev. Growth Differ. 50, S113–S118.
Namba, K., Sugihara, I., and Hashimoto, M. (2011). Close correlation between the birth date of Purkinje cells and the longitudinal compartmentalization of the mouse adult cerebellum. J. Comp. Neurol. 519, 2594–2614.
Neudert, F., and Redies, C. (2008). Neural circuits revealed by axon tracing and mapping cadherin expression in the embryonic chicken cerebellum. J. Comp. Neurol. 509, 283–301.
Nishida, K., Hoshino, M., Kawaguchi, Y., and Murakami, F. (2010). Ptf1a directly controls expression of immunoglobulin superfamily molecules Nephrin and Neph3 in the developing central nervous system. J. Biol. Chem. 285, 373–380.
Novak, A., Guo, C., Yang, W., Nagy, A., and Lobe, C. G. (2000). Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155.
Nunzi, M. G., Grillo, M., Margolis, F. L., and Mugnaini, E. (1999). Compartmental organization of Purkinje cells in the mature and developing mouse cerebellum as revealed by an olfactory marker protein-lacZ transgene. J. Comp. Neurol. 404, 97–113.
Oberdick, J., Schilling, K., Smeyne, R. J., Corbin, J. G., Bocchiaro, C., and Morgan, J. I. (1993). Control of segment-like patterns of gene expression in the mouse cerebellum. Neuron 10, 1007–1018.
Ohshima, T., and Mikoshiba, K. (2002). Reelin signaling and Cdk5 in the control of neuronal positioning. Mol. Neurobiol. 26, 153–166.
Ozol, K. O., and Hawkes, R. (1997). The compartmentation of the granular layer of the cerebellum. Histol. Histopathol. 12, 171–184.
Ozol, K., Hayden, J. M., Oberdick, J., and Hawkes, R. (1999). Transverse zones in the vermis of the mouse cerebellum. J. Comp. Neurol. 412, 95–111.
Pakan, J. M. P., Iwaniuk, A. N., Wong-Wylie, D. R., Hawkes, R., and Marzban, H. (2007). Purkinje cell compartmentation as revealed by zebrin II expression in the cerebellar cortex of pigeons (Columba livia). J. Comp. Neurol. 501, 619–630.
Paradies, M. A., and Eisenman, L. M. (1993). Evidence of early topographic organization in the embryonic olivocerebellar projection: a model system for the study of pattern formation processes in the central nervous system. Dev. Dyn. 197, 125–145.
Paradies, M. A., Grishkat, H., Smeyne, R. J., Oberdick, J., Morgan, J. I., and Eisenman, L. M. (1996). Correspondence between L7-lacZ-expressing Purkinje cells and labeled olivocerebellar fibers during late embryogenesis in the mouse. J. Comp. Neurol. 374, 451–466.
Pascual, M., Abasolo, I., Mingorance-Le Meur, A., Martinez, A., Del Rio, J. A., Wright, C. V., Real, F. X., and Soriano, E. (2007). Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc. Natl. Acad. Sci. U.S.A. 104, 5193–5198.
Pozzoli, O., Bosetti, A., Croci, L., Consalez, G. G., and Vetter, M. L. (2001). Xebf3 is a regulator of neuronal differentiation during primary neurogenesis in Xenopus. Dev. Biol. 233, 495–512.
Redies, C., Neudert, F., and Lin, J. (2011). Cadherins in cerebellar development: translation of embryonic patterning into mature functional compartmentalization. Cerebellum 10, 393–408.
Reeber, S. L., and Sillitoe, R. V. (2011). Patterned expression of a cocaine- and amphetamine-regulated transcript peptide reveals complex circuit topography in the rodent cerebellar cortex. J. Comp. Neurol. 519, 1781–1796.
Rice, D. S., Sheldon, M., D'Arcangelo, G., Nakajima, K., Goldowitz, D., and Curran, T. (1998). Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development 125, 3719–3729.
Rivkin, A., and Herrup, K. (2003). Development of cerebellar modules: extrinsic control of late-phase zebrin II pattern and the exploration of rat/mouse species differences. Mol. Cell. Neurosci. 24, 887–901.
Rouse, R. V., and Sotelo, C. (1990). Grafts of dissociated cerebellar cells containing Purkinje cell precursors organize into zebrin I defined compartments. Exp. Brain Res. 82, 401–407.
Ruthazer, E. S., and Cline, H. T. (2004). Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J. Neurobiol. 59, 134–146.
Salsano, E., Croci, L., Maderna, E., Lupo, L., Pollo, B., Giordana, M. T., Consalez, G., and Finocchiaro, G. (2007). Expression of the neurogenic basic helix-loop-helix transcription factor NEUROG1 identifies a subgroup of medulloblastomas not expressing ATOH1. Neuro Oncol. 9, 298–307.
Sarna, J., and Hawkes, R. (2003). Patterned Purkinje cell death in the cerebellum. Prog. Neurobiol. 70, 473–507.
Sarna, J. R., Marzban, H., Watanabe, M., and Hawkes, R. (2006). Complementary stripes of phospholipase Cß3 and Cß4 expression by Purkinje cell subsets in the mouse cerebellum. J. Comp. Neurol. 496, 303–313.
Seil, F. J., Johnson, M. L., and Hawkes, R. (1995). Molecular compartmentation expressed in cerebellar cultures in the absence of neuronal activity and neuron-glial interactions. J. Comp. Neurol. 356, 398–407.
Sentürk, A., Pfennig, S., Weiss, A., Burk, K., and Acker-Palmer, A. (2011). Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature 472, 356–360.
Sheldon, M., Rice, D. S., D'Arcangelo, G., Yoneshima, H., Nakajima, K., Mikoshiba, K., Howell, B. W., Cooper, J. A., Goldowitz, D., and Curran, T. (1997). Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389, 730–733.
Sillitoe, R. V., Chung, S-H., Fritschy, J. M., Hoy, M., and Hawkes, R. (2008). Golgi cell dendrites are restricted by Purkinje cell stripe boundaries in the adult mouse cerebellar cortex. J. Neurosci. 28, 2820–2826.
Sillitoe, R. V., Gopal, N., and Joyner, A. L. (2009). Embryonic origins of zebrin II parasagittal stripes and establishment of topographic Purkinje cell projections. Neuroscience 162, 574–588.
Sillitoe, R. V., and Hawkes, R. (2002). Whole mount immunohistochemistry: a high throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 50, 235–244.
Sillitoe, R. V., and Joyner, A. L. (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 23, 549–577.
Sillitoe, R. V., Marzban, H., Larouche, M., Zahedi, S., Affanni, J., and Hawkes, R. (2005). Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Prog. Brain Res. 148, 283–297.
Sillitoe, R. V., Vogel, M. W., and Joyner, A. L. (2010). Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J. Neurosci. 30, 10015–10024.
Simeone, A. (2000). Positioning the isthmic organizer where Otx2 and Gbx2 meet. Trends Genet. 16, 237–240.
Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71.
Sotelo, C., and Chédotal, A. (2005). Development of the olivocerebellar system: migration and formation of cerebellar maps. Prog. Brain Res. 148, 1–20.
Sotelo, C., and Wassef, M. (1991). Cerebellar development: afferent organization and Purkinje cell heterogeneity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 331, 307–313.
Srinivas, S., Watanabe, T., Lin, C. S., William, C. M., Tanabe, Y., Jessell, T. M., and Costantini, F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4.
Strasser, V., Fasching, D., Hauser, C., Mayer, H., Bock, H. H., Hiesberger, T., Herz, J., Weeber, E. J., Sweatt, J. D., Pramatarova, A., Howell, B., Schneider, W. J., and Nimpf, J. (2004). Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 24, 1378–1386.
Sugihara, I. (2011). Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase c expression. Cerebellum 10, 449–463.
Takeda, T., and Maekawa, K. (1989). Transient direct connection of vestibular mossy fibers to the vestibulocerebellar Purkinje cells in early postnatal development of kittens. Neuroscience 32, 99–111.
Tano, D., Napieralski, J. A., Eisenman, L. M., Messer, A., Plummer, J., and Hawkes, R. (1992). Novel developmental boundary in the cerebellum revealed by zebrin expression in the Lurcher (Lc/+) mutant mouse. J. Comp. Neurol. 323, 128–136.
Terada, N., Banno, Y., Ohno, N., Fujii, Y., Murate, T., Sarna, J., Hawkes, R., Zea, Z., Baba, T., and Ohno, S. (2004). Compartmentation of the mouse cerebellar cortex by sphingosine kinase. J. Comp. Neurol. 469, 119–127.
Thomas, K. R., and Capecchi, M. R. (1990). Targeted disruption of the murine int-1 proto- oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 346, 847–850.
Tissir, F., and Goffinet, A. M. (2003). Reelin and brain development. Nat. Rev. Neurosci. 4, 496–505.
Trommsdorff, M., Gotthardt, M., Hiesberger, T., Shelton, J., Stockinger, W., Nimpf, J., Hammer, R. E., Richardson, J. A., and Herz, J. (1999). Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701.
Vandaele, S., Nordquist, D. T., Feddersen, R. M., Tretjakoff, I., Peterson, A. C., and Orr, H. T. (1991). Purkinje cell protein-2 regulatory regions and transgene expression in cerebellar compartments. Genes Dev. 5, 1136–1148.
Vue, T. Y., Aaker, J., Taniguchi, A., Kazemzadeh, C., Skidmore, J. M., Martin, D. M., Martin, J. F., Treier, M., and Nakagawa, Y. (2007). Characterization of progenitor domains in the developing mouse thalamus. J. Comp. Neurol. 505, 73–91.
Wadiche, J. I., and Jahr, C. E. (2005). Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat. Neurosci. 10, 1329–1334.
Wassef, M., and Joyner, A. L. (1997). Early mesencephalon/metencephalon patterning and development of the cerebellum. Perspect. Dev. Neurobiol. 5, 3–16.
Wassef, M., Sotelo, C., Thomasset, M., Granholm, A-C., Leclerc, N., Rafrafi, J., and Hawkes, R. (1990). Expression of compartmentation antigen zebrin I in cerebellar transplants. J. Comp. Neurol. 294, 223–234.
Wassef, M., Zanetta, J. P., Brehier, A., and Sotelo, C. (1985). Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev. Biol. 111, 129–137.
Yang, H., Jensen, P., and Goldowitz, D. (2002). The community effect and Purkinje cell migration in the cerebellar cortex: analysis of scrambler chimeric mice. J. Neurosci. 22, 464–470.
Zhao, Y., Kwan, K-M., Mailloux, C., Lee, W-K., Grinberg, A., Wurst, W., Behringer, R., and Westphal, H. (2007). LIM-homeodomain proteins Lhx1 and Lhx5, and their cofactors Ldb1, control Purkinje cell differentiation in the developing cerebellum. Proc. Natl. Acad. Sci. U.S.A. 104, 13182–13186.
Keywords: Purkinje cell, ventricular zone, zebrin, EBF2, neurogenin, stripe
Citation: Dastjerdi FV, Consalez GG and Hawkes R (2012) Pattern formation during development of the embryonic cerebellum. Front. Neuroanat. 6:10. doi: 10.3389/fnana.2012.00010
Received: 09 December 2011; Accepted: 14 March 2012;
Published online: 04 April 2012.
Edited by:
José A. Armengol, University Pablo de Olavide, SpainReviewed by:
Ferdinando Rossi, University of Turin, ItalyIsabelle Dusart, Centre National de la Recherche Scientifique, France
Copyright: © 2012 Dastjerdi, Consalez and Hawkes. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: G. G. Consalez, Division of Neuroscience, DIBIT1, 3A2 room 36, San Raffaele Scientific Institute, Via Olgettina 58, 20132 Milano, Italy. email: g.consalez@hsr.it
R. Hawkes, Faculty of Medicine, Department of Cell Biology and Anatomy, University of Calgary, 3330 Hospital Drive N.W., Calgary, AB T2N 4N1, Canada. e-mail: rhawkes@ucalgary.ca