- Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA, USA
Metalloporphyrins are structural analogs of heme and their potential use in the management of neonatal hyperbilirubinemia has been the subject of considerable research for more than three decades. The pharmacological basis for using this class of compounds to control bilirubin levels is the targeted blockade of bilirubin production through the competitive inhibition of heme oxygenase (HO), the rate-limiting enzyme in the bilirubin production pathway. Ongoing research continues in the pursuit of identifying ideal metalloporphyrins, which are safe and effective, by defining therapeutic windows and targeted interventions for the treatment of excessive neonatal hyperbilirubinemia.
Introduction
Metalloporphyrins (Mps) and their potential use in the management of neonatal hyperbilirubinemia has been the subject of considerable research for more than three decades. The therapeutic approach for using this class of anti-hyperbilirubinemia drugs is the targeted blockade of bilirubin production through competitive inhibition of heme oxygenase (HO), the key enzyme in the heme degradative pathway (Tenhunen et al., 1968).
Neonatal jaundice is one of the most common problems for newborn infants during the first weeks of life, affecting approximately 60–70% of term babies and almost all premature babies (American Academy of Pediatrics, 2004). Hyperbilirubinemia is due to a transitional imbalance between bilirubin production and elimination processes. To date, the most commonly used treatments of pathologic bilirubin levels only remove bilirubin that has already accumulated in the body, by initiating phototherapy or, in the extreme cases, performing an exchange transfusion (American Academy of Pediatrics, 2004; Stevenson and Wong, 2010). However, the total serum or plasma bilirubin (TB) concentration at which to begin phototherapy is still controversial and difficult to define to a precise number that can be applied universally to all newborn infants. Instead, it differs according to age (term or preterm), genetic, and ethnic backgrounds, hepatic conjugation capacity, albumin binding, blood/tissue distribution of bilirubin, physiological homeostasis, presence of pre-existing hemolytic conditions, and also individual susceptibility to bilirubin toxicity. In addition, the use of intravenous immunoglobulin (IVIG) has been shown to be effective in reducing TB levels in infants with ABO hemolytic disease, reducing the degree of hemolysis by stabilizing red blood cells (RBC; American Academy of Pediatrics, 2004). Adverse effects related to IVIG therapy include fever, allergic reactions, rebound hemolysis, and fluid overload.
The pharmacologic use of Mps for controlling bilirubin production rates may be strategically a more effective approach (Drummond and Kappas, 1981, 1982a; Stevenson et al., 1989). Its efficacy as a therapeutic and preventive treatment strategy in the management of neonatal hyperbilirubinemia has been confirmed in a large number of animal and clinical studies. In spite of this, Mps still have not left the clinical study stage for their actual application in human neonates, mainly due to the photosensitizing potential of these compounds. This property becomes particularly problematic in preterm infants, a very vulnerable patient group, with thin, transparent skin, reduced antioxidant capacity, a high surface to volume ratio, and frequent potential exposure to phototherapy (Morris et al., 2008). Moreover, a selective review of only available randomized, controlled clinical trials, comparing Mp treatment with placebo or conventional treatments, shows that the combined number of subjects studied is actually relatively small and the authors conclude that more studies are still needed to evaluate the reduction of bilirubin-induced neurological dysfunction (BIND) compared to other treatments (Suresh et al., 2003). Also the short- and long-term effects of Mps, such as the possible release, accumulation, and toxicity of the metal moiety (Hiles, 1974; Maines, 1992), and effects on oxygen radical diseases of prematurity (e.g., bronchopulmonary dysplasia, intraventricular hemorrhage, patent ductus arteriosus, retinopathy of prematurity, and necrotizing enterocolitis) need to be further elucidated (Suresh et al., 2003).
Ongoing research continues in the pursuit of identifying ideal Mps, and, most importantly, of allaying concerns about toxicity, through defining therapeutic windows, and safe treatment strategies of potential candidate compounds.
Neonatal Hyperbilirubinemia
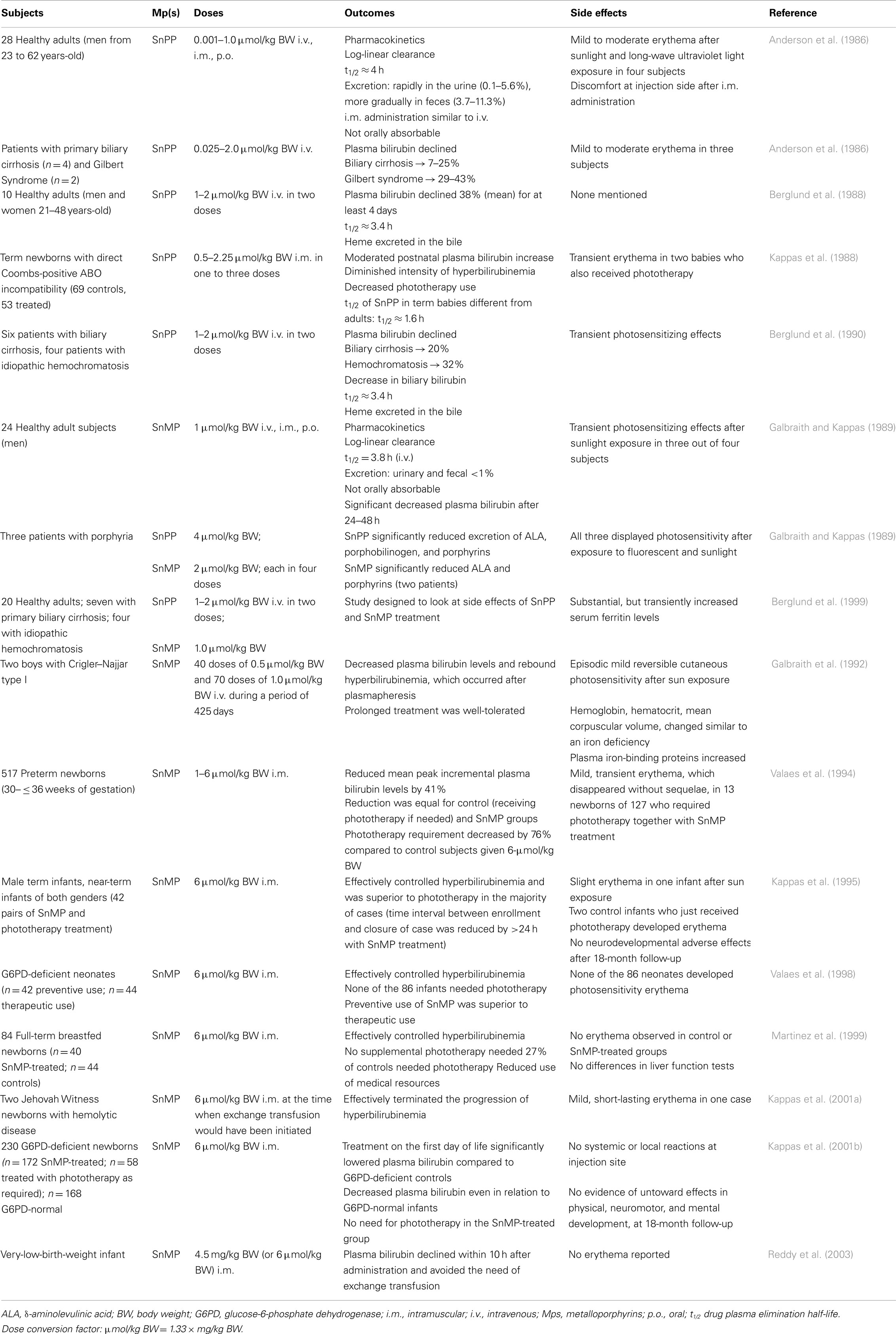
When bilirubin levels in circulation become excessive, it may lead to bilirubin deposition in the brain, and if left untreated, cause severe and permanent neurological damage (or BIND; Penn et al., 1994; Gourley, 1997; Govaert et al., 2003; Stevenson et al., 2011). Bilirubin derives from the degradation of heme, the prosthetic group of hemoglobin, and other hemoproteins, which occurs primarily in the spleen and liver. In this enzymatic pathway, HO catalyzes the rate-limiting oxidation of heme to release equimolar amounts of free iron (Fe2+), carbon monoxide (CO), and biliverdin. The latter is subsequently and rapidly reduced to bilirubin by biliverdin reductase. Both reactions require NADPH as a reducing agent (Tenhunen et al., 1968, 1970; Figure 1).
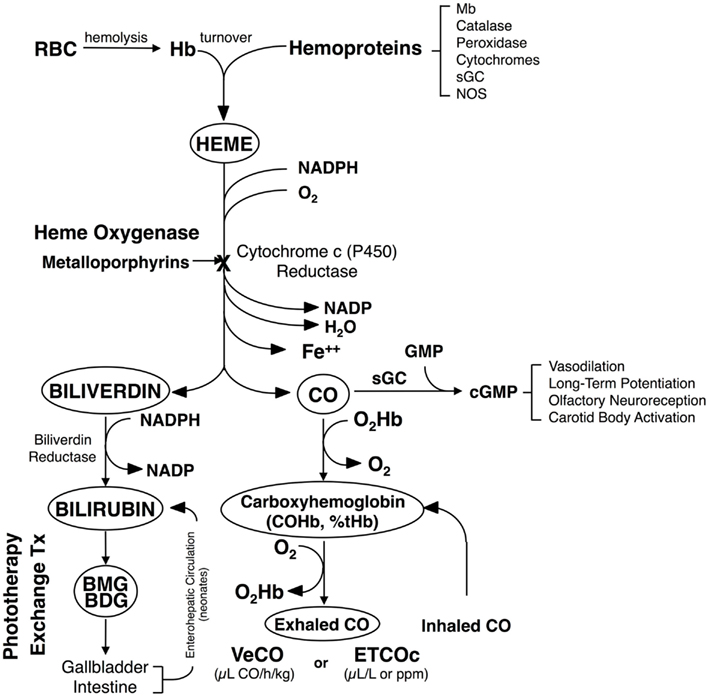
Figure 1. Heme degradation pathway. The turnover of hemoglobin (Hb) and other hemoproteins yields heme. This heme is metabolized to equimolar quantities of carbon monoxide (CO), iron (Fe2+), and biliverdin. Biliverdin is subsequently reduced to form bilirubin. CO is bound to circulating red blood cells (RBC) and is excreted through the lungs, where it can be measured as the rate of total body CO excretion (VeCO) or as a concentration in end-tidal breath, corrected for ambient CO (ETCOc). Modified from Vreman et al. (2001).
Once in circulation, bilirubin becomes bound to albumin and is then transported to the liver, where it is conjugated to mono- and diglucuronic acids by uridine diphosphoglucuronate glucuronosyltransferase (UGT). Being water-soluble, the conjugated bilirubin is excreted in the bile and finally eliminated from the body through the bowel. However, the glucuronides are relatively unstable and can be hydrolyzed to unconjugated bilirubin, which can be absorbed by the intestinal mucosa, and re-enters the circulation (enterohepatic circulation). Besides increased enterohepatic reabsorption of bilirubin, decreased hepatic uptake, and conjugation, are the major factors contributing to the impaired elimination of bilirubin observed after birth (Kaplan et al., 2011).
The Rationale for the Use of Metalloporphyrins
The bilirubin production rate of newborn infants is normally two to three times higher than that of adults, which is mainly due to an increased circulating RBC mass and a shortened RBC lifespan, and hence an increase in RBC turnover (Stevenson et al., 1994). Since all newborn infants have impaired bilirubin clearance, any condition causing an increased production rate, such as hemolysis, represents a serious risk. This unconjugated hyperbilirubinemia occurs primarily in infants with isoimmune hemolytic diseases caused by blood group incompatibilities between mother and fetus, such as Rh isoimmunization and ABO incompatibility, or by glucose-6-phosphate dehydrogenase (G6PD) deficiency. When uncontrolled, it can lead to the development BIND. Normally, peak TB concentrations in term infants range from 5 to 6 mg/dL (86–103 μmol/L) at 48–120 h after birth in Caucasian and African-American infants and from 10 to 14 mg/dL (171–239 μmol/L) at 72–120 h after birth in Asian-American infants. In premature infants, TB levels peak by the fifth day of life, reaching 10–12 mg/dL (171–205 μmol/L; Kaplan et al., 2011).
In addition to an immature and temporally insufficient bilirubin clearance and a physiological increased production in newborn infants, genetic vulnerabilities, such as polymorphisms in the UGT1A1 promoter (low bilirubin eliminator) and/or in the HO-1 promoter (less GT repeats equals high bilirubin producer) and G6PD deficiency (high bilirubin producer) can place the infant at high risk for developing hyperbilirubinemia (Cohen et al., 2010). The use of CO detection technologies, e.g., end-tidal breath CO measurements, corrected for ambient CO (ETCOc; Vreman et al., 1994, 1996, 1999), or total body excretion rates of CO (VeCO), can provide estimates of total CO production, which is a direct index of bilirubin production (Stevenson et al., 1979) under steady state conditions, where the CO produced from other sources (15–20%), such as lipid peroxidation or photo-oxidation, are controlled for (Dercho et al., 2006). Thus, the antenatal diagnoses of genetic predispositions and the use of ETCOc could allow the identification of high bilirubin producers, who could be targeted for treatment with Mps before TB levels become excessive. No clinical device is presently commercially available. However, a prototype instrument (Co-Sense, Capnia, Inc., Palo Alto, CA, USA) is currently evaluated for use in clinical studies.
Although phototherapy can be regarded as a “drug” for the treatment of hyperbilirubinemia, its therapeutic use not only differs from “classic” pharmaceuticals, but also has several characteristic limitations. Ideally, phototherapy devices should deliver light with: an emission spectrum between 400 and 520 nm (blue–green; Vreman et al., 2004); an irradiance footprint which exposes at least one entire horizontal body surface plane; an irradiance (intensity) level of ≥30 μW/cm2/nm; and an optimized duration of exposure (American Academy of Pediatrics, 2004; Maisels and McDonagh, 2008). Compared to a traditional drug, phototherapy is a non-specific, instead of a targeted, treatment strategy and it only removes bilirubin, which already has been formed. Moreover, its therapeutic dose is not a fixed number, but a still debated light intensity range, which is dependent on an accurate measurement of the irradiance of a given light source, often problematic itself (Vreman et al., 2008). Also, the spectral characteristics of phototherapy devices are quite different and may account for variations in efficacy and safety (Vreman et al., 2008).
Nonetheless, phototherapy is generally considered safe, effective, and simple to administer and therefore used routinely in the clinical setting. Recently, however, concerns have been raised about its safe use in extremely low birth weight (ELBW) infants (501–750 g). Because their antioxidant capacity is often limited, phototherapy has been shown to promote oxidative stress in this patient group (Gathwala and Sharma, 2002). Evidence of injurious effects of phototherapy has been found in a National Institute of Child Health and Development trial comparing the use of aggressive vs. conservative phototherapy. Although there was a significant decrease in neurodevelopmental impairment in ELBW infants, post hoc analyses revealed an increased mortality in this cohort, which did not reach statistical significance (Morris et al., 2008). Moreover, some studies have reported that re-opening of the ductus arteriosus has been associated with phototherapy use for premature infants (Barefield et al., 1993; Benders et al., 1999);whereas, others failed to show this correlation (Scheidt et al., 1987; Travadi et al., 2006).
A more strategic approach may be through the direct inhibition of bilirubin production using Mps. Targeting high bilirubin producers (such as infants with hemolytic diseases, the most common cause of pathological unconjugated hyperbilirubinemia) would be the most beneficial application for Mps, and therefore may reduce or eliminate the need for exchange transfusion in this infant population. The effectiveness in reducing severe hemolytic hyperbilirubinemia and thereby preventing the need for an exchange transfusion has been described in a case report using SnMP (Reddy et al., 2003). Additionally, phototherapy has been shown to have limited effect in modulating elevated TB levels due to Coombs-positive hemolytic disease and cannot be considered as a substitute for exchange transfusion (Maurer et al., 1985). It is also conceivable that hyperbilirubinemia treatment with Mps could be beneficial for premature infants, which have very thin skin, thus light can penetrate deeper into tissue and cause photo-oxidative injury (Vreman et al., 2004; Hintz et al., 2011). This effect might be reduced with Mps treatment, if they are used alone and not in combination with phototherapy. A clinical study by Valaes et al. (1994), using SnMP to control TB in premature babies described no adverse effects of SnMP treatment alone (without phototherapy). However, to state unequivocally that the use of Mps is advantageous over phototherapy for these ELBW infants, who appear to be more sensitive to the adverse effects of phototherapy, is complex and mostly speculative.
Pharmacodynamics of Metalloporphyrins
Porphyrins (Greek for “purple”) are a class of tetrapyrrole macrocycles with a skeleton of 16-atom rings containing four nitrogen atoms. The porphine free base has 11 double bounds and can easily be transformed into an Mp by replacing the inner two pyrrole protons with a metal ion. The porphyrin ring itself has a planar structure due to the high number of double bonds (Fleischer, 1970). Depending on the side chains and central metal ion, a large number and variety of Mps are possible (Figure 2).
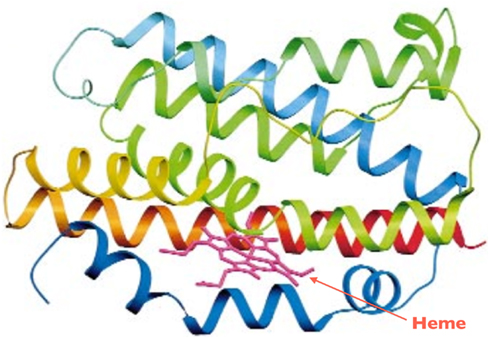
Figure 2. Ribbon diagram of HO-1. The N-terminus is blue and the C-terminus is red, with green in the middle. Heme is shown by the arrow. Adapted from Schuller et al. (1999).
The inhibition of HO by Mps was initially reported in 1981 by Maines (1981) and Drummond and Kappas (1981). Zinc (Drummond and Kappas, 1981; Maines, 1981), tin (Drummond and Kappas, 1981; Maines, 1981), and manganese protoporphyrin (Drummond and Kappas, 1981; ZnPP, SnPP, MnPP, respectively) were the first Mps observed to be competitive inhibitors for HO in the liver (Drummond and Kappas, 1981; Maines, 1981), spleen (Drummond and Kappas, 1981; Maines, 1981), kidney (Drummond and Kappas, 1981; Maines, 1981), and skin tissue (Drummond and Kappas, 1981) in vitro and in vivo. These compounds have a much higher binding affinity (e.g., SnPP: Ki = 0.011 μM in rat spleen tissue; Drummond and Kappas, 1981) than heme to HO-1 and HO-2 (Km = 0.24 and 0.67 μM, respectively; Ryter et al., 2006). They are not oxidatively degraded because they have no oxygen-binding capacity. Chromium protoporphyrin (CrPP) has also been shown to inhibit HO activity in vitro (rat and human spleen) and in vivo (rat liver and spleen) and thus prevent hyperbilirubinemia in neonatal rats (Drummond and Kappas, 1982b). Protoporphyrins with cobalt (Co; Drummond and Kappas, 1981; Maines, 1981), iron (Fe; Drummond and Kappas, 1981; Maines, 1981), or cadmium (Cd; Drummond and Kappas, 1981) as central metals have been found to induce HO; but only iron containing Mps, such as heme (FePP), act as actual substrates. CoPP is a unique Mp exhibiting a dualism: significantly inhibiting HO activity in vitro (Maines, 1981; Yoshinaga et al., 1982) and enhancing HO activity in vivo (Drummond and Kappas, 1981; Maines, 1981) due to its strong activation of HO-1 gene expression (Maines, 1981; Kappas and Drummond, 1986; Shan et al., 2006). Subsequent studies showed that iron deuteroporphyrin is also significantly metabolized by liver tissue homogenates in an HO-like mechanism (Vreman et al., 1993). In contrast, HO activity is largely unaffected by protoporphyrins with nickel (Ni), copper (Cu), and magnesium (Mg) as central atoms (Drummond and Kappas, 1981).
SnPP has been shown to be effective toward inhibiting HO activity in vivo and in vitro, preventing the development of neonatal hyperbilirubinemia shortly after birth in the rat (Drummond and Kappas, 1981, 1982a) and rhesus neonate (Cornelius and Rodgers, 1984). A decrease in TB has also been demonstrated in adult mice with congenital forms of hemolytic anemia (Sassa et al., 1983), in the postnatal suckling rat with heme- or δ-aminolevulinic acid-induced hyperbilirubinemia (Drummond and Kappas, 1984), in the bile-duct ligated rat (Kappas et al., 1984; McMillan et al., 1987), and in a number of clinical studies with human adults (Anderson et al., 1986; Berglund et al., 1988, 1990) or newborns (Kappas et al., 1988). However, studies showing that SnPP is a photosensitizer of bilirubin destruction in vitro (McDonagh and Palma, 1985), and phototoxic in vivo (Hintz et al., 1990) led to its abandonment for use in human infants. Nonetheless, it should be noted that the photosensitizing properties of SnPP can be advantageous, such as in the photodynamic treatment of psoriasis (Emtestam et al., 1989).
The naturally occurring ZnPP appeared to be especially attractive as it is relatively inert to light activation and thus has no photosensitizing/phototoxic effects in vivo (Hintz et al., 1990; Labbe et al., 1999). Early studies by Maines showed that the subcutaneous (s.c.) application of ZnPP at a dose of 40 μmol/kg body weight (BW) was effective in inhibiting HO activity in neonatal rats and neonatal rhesus monkeys (Maines, 1981; Qato and Maines, 1985). The same ZnPP dose given intravenously (i.v.) also significantly reduced total body VeCO in rhesus neonates (Rodgers et al., 1990), and, in the newborn rhesus with iatrogenic hemolysis, VeCO, carboxyhemoglobin, TB, and spleen HO activity (Vreman et al., 1990b).
Further research demonstrated that tin mesoporphyrin (SnMP; Drummond et al., 1987), chromium mesoporphyrin (CrMP; Vreman et al., 1993), and zinc deuteroporphyrin IX bis glycol (ZnBG; Martasek et al., 1988; Chernick et al., 1989; Vreman et al., 1991) are also attractive candidates for use in the treatment of neonatal jaundice primarily due to their high potency.
Three HO isoenzymes have been identified to date (Maines et al., 1986; Cruse and Maines, 1988; McCoubrey and Maines, 1994). Whereas HO-1 and HO-2 actively catalyze heme to biliverdin and CO, HO-3 is regarded as a pseudogene of HO-2 and its functional activity is still uncertain (McCoubrey et al., 1997; Ryter et al., 2006).
The HO-2 isoform (∼36 kDa) is constitutively expressed in all tissues, primarily expressed in the brain and highest in the testes (Trakshel et al., 1986, 1988). Conversely, under homeostatic conditions, most tissues express HO-1 at relatively low levels, but can respond to stress with rapid transcriptional activation of the HO-1 gene. The spleen and reticuloendothelial cells in the liver and bone marrow degrade senescent RBCs, and thus highly express HO-1 under basal conditions (Ryter et al., 2006). The catalytic pocket of the HO-1 enzyme with its substrate heme is shown in Figure 3.
Figure 3. Basic porphyrin IX structure with central metal and two ring substitution sites (R). Oxidation of susceptible porphyrins, catalyzed by HO, occurs at the α-position to yield a tetrapyrrole. Modified from Vreman et al. (2001).
Different enzyme kinetics and heme Km values are known for HO-1 and HO-2 (Km = 0.24 and 0.67 μM, respectively; Maines et al., 1986; Ryter et al., 2006) and make varying interactions of Mps with HO-1 and HO-2 very plausible. A recent study by Wong et al. (2011) characterized the in vitro potency of a variety of Mps toward inhibiting HO-1 and HO-2 isoenzymes, using rat and mouse spleen and brain tissue, respectively, as sources of the isoenzymes. SnMP, CrMP, and ZnBG were shown to have the highest potency toward suppression of HO-1 and HO-2 activities. Interestingly, all Mps more selectively inhibit the HO-2 isoenzyme over HO-1. However, CrMP had the highest selectivity toward HO-1 inhibition of all Mps tested, followed by ZnBG and ZnPP. SnPP appeared to be most selective for HO-2. It is conceivable that inhibition of the inducible HO-1 is preferable in a clinical setting because its activity increases in response to hemolytic conditions. Moreover, a strong and a prolonged inhibition of HO-2 may be detrimental as HO-2 is the predominant form in most organs under homeostatic conditions. An early report using rats also describes a selectivity of SnPP toward HO-2 inhibition in addition to a dramatic disruption of the integrity of the HO-2 protein, which may add to the significant suppression of TB formation by SnPP (Maines and Trakshel, 1992a). Similar results regarding the potency of various Mps have also been described by Vreman et al. (1993) comparing their efficacy to inhibit rat liver HO activity, with HO-1 and HO-2 equally contributing to the total HO activity under non-stimulated conditions. Whereas CrMP was most effective in inhibiting total liver HO activity in vitro, SnPP, SnMP, ZnPP, and ZnMP appeared nearly equally potent.
In vivo, the efficacy of Mps is dependent on several factors: route of administration, plasma and tissue distribution, and also underlies species differences. Although ZnPP appeared less potent than SnPP, both Mps effectively suppressed HO activity in liver, spleen, kidney (ZnPP only rat tissue), and TB levels with a long duration of action (ZnPP up to 12 days in rhesus neonates; SnPP up to 42 days in rats; Drummond and Kappas, 1981, 1982a; Maines, 1981; Cornelius and Rodgers, 1984; Qato and Maines, 1985; Rodgers et al., 1990). Variations of the porphyrin side chain enhanced the effectiveness toward HO inhibition 10-fold for SnMP compared to SnPP (Drummond et al., 1987). However, ZnBG seems to be one of the most potent inhibitors in vivo, but with a short duration of action (Vreman et al., 1991; He et al., 2011).
Besides having HO isoform selectivity, Mps also differ in tissue distribution, potency, photosensitivity, and other side effects. Although the bioavailability and photosensitivity of Mps are dependent on certain aspects of the Mps structure, as the hydrophilicity of the side chains, and the electronic configuration of the metal atom, in general, there is no set pattern, which can allow one to predict the behavior of a given Mp in vivo.
Clinical Studies
To date, clinical efficacy studies have only been performed with SnPP and later with SnMP. An early human trial with a limited number of adult subjects (n = 6) given SnPP (0.25–2.0 μmol/kg BW i.v.) demonstrated a decrease in TB from 7 to 23% in patients with cholestasis secondary to primary biliary cirrhosis, and 29–43% in patients with Gilbert syndrome (Anderson et al., 1986). However, some treated subjects (4/28 normal subjects, and 3 with hyperbilirubinemia) developed mild to moderate transient erythema and conjunctival irritation after sunlight exposure. In term newborns with hyperbilirubinemia due to direct Coombs-positive ABO incompatibility, SnPP diminished TB compared to control infants with significant differences in the incremental changes in TB concentration after two or three intramuscular (i.m.) doses of 0.75 μmol/kg BW. The effect of a single dose of 0.5 μmol SnPP/kg BW did not reach statistical significance. The need for phototherapy was reduced in the SnPP-treatment group, but following light treatment, 2 of 24 infants treated with SnPP developed transient erythema (Kappas et al., 1988). In several other studies with healthy human subjects or patients with hepatic dysfunction affecting heme metabolism or bilirubin conjugation, SnPP administration was described as being relatively innocuous, despite causing transient photosensitizing effects after single or multiple applications (Kappas et al., 1984; Berglund et al., 1988, 1990; Galbraith et al., 1992).
Since SnMP has been shown to be at least 10-fold more potent than SnPP in inhibiting HO activity (Drummond et al., 1987), clinical studies were pursued with the expectation that its high potency would allow for its use at much lower doses, and therefore its photosensitizing effects would be minimized or maybe even eliminated. In spite of this rationale, SnMP was used in a dose of 1–6 μmol/kg BW, which was equal to or higher than the dose of SnPP used in earlier studies. Clinical studies in human preterm (Valaes et al., 1994), full-term (Martinez et al., 1999), and near-term newborns (Kappas et al., 1995) showed that SnMP substantially moderated the course of hyperbilirubinemia, significantly decreasing the mean peak incremental TB concentration (Valaes et al., 1994), phototherapy use (Valaes et al., 1994; Kappas et al., 1995; Martinez et al., 1999), and length of hospital stay (Kappas et al., 1995; Martinez et al., 1999) compared to controls. However, no significant difference in the TB concentrations was shown between control groups, who mostly received phototherapy vs. SnMP groups (Kappas et al., 1995). Several infants who needed phototherapy in addition to SnMP treatment developed transient erythema similar to that observed in SnPP-treated newborns (Valaes et al., 1994; Kappas et al., 1995). These studies used “special blue” Philips F20T12/BB fluorescent tubes, with an emission spectrum (maximum intensity 440–460 nm), which does not extend into the Soret peak as full spectrum white light does (Valaes et al., 1994). Delaney et al. (1988) demonstrated that the triplet lifetime of SnPP decreases ∼95% when excited at 450 nm, which presumably also decreases its phototoxicity in the emission range of the special blue lamp compared to full spectrum light from any source. Moreover, the irradiance of these earlier studies was relatively low (12–14 μW/cm2/nm) compared to that recommended in the 2004 American Academy of Pediatrics (AAP) practice guideline (≥30 μW/cm2/nm; American Academy of Pediatrics, 2004). The phototoxicity of Mps appears to be strongly dependent on the irradiance and spectral quality of the light source (Schulz et al., 2012), and therefore the occurrence of more worrisome photosensitizing side effects should not be excluded. In G6PD-deficient newborns, a preventive or therapeutic SnMP administration supplanted the need for phototherapy, but SnMP showed no advantages over phototherapy in its effectiveness in controlling TB levels (Valaes et al., 1998).
Suresh et al. (2003) reviewed the available data from clinical studies with Mps in order to determine the efficacy of Mps in reducing TB levels, the need for phototherapy or exchange transfusion, and the incidence of BIND in neonates with unconjugated hyperbilirubinemia. We have summarized the clinical studies described to date in Table 1. A multicenter clinical trial conducted by InfaCare Pharmaceutical Corporation evaluating the long-term effects of SnMP (Stannsoporfin) was begun in 2008 and the results from this study are still pending.
Side Effects
A question that surfaced early was the fate of the potential cytotoxicity of heme after blockade of its metabolism. Studies performed using bile-cannulated rats have demonstrated that after administration of exogenous heme or heat-damaged RBCs together with SnPP, the amount of heme excreted into the bile markedly increased; whereas, the biliary output of bilirubin diminished (Kappas et al., 1985; Hintz et al., 1987). Therefore, it appears that no accumulation of the cytotoxic and irritant heme occurs after HO inhibition. An enhanced excretion of heme in the bile after SnPP-mediated HO inhibition has also been shown in a study with 10 healthy adults, using duodenal intubation (Berglund et al., 1988).
Mps have been found to also interact with other heme-containing enzyme systems, such as nitric oxide synthase (NOS; Luo and Vincent, 1994; Meffert et al., 1994), soluble guanylyl cyclase (sGC; Ignarro et al., 1984; Grundemar and Ny, 1997), and cytochrome P450 (CYP450; Drummond et al., 1989; Trakshel et al., 1992). They also affect hematopoiesis (Maines and Trakshel, 1992b; Lutton et al., 1997), steroidogenesis (Maines and Trakshel, 1992b; Drummond et al., 1996), and the iron status of the body (Kappas et al., 1993; Berglund et al., 1999). However, the most prominent and concerning side effect is the photosensitizing property of the majority of Mps.
Photosensitivity
It is understood that the Mp-sensitized photodynamic damage is mainly caused by the absorption of light at wavelengths of 400 (Soret band), 540, and 580 nm, the peak absorptions of Mps. This subsequently causes the formation of triplet excited states, long triplet lifetimes, and high quantum yields for sensitizing the formation of singlet oxygen, which reacts with biological substrates (e.g., amino acids, guanine bases of DNA and RNA, and unsaturated lipids, including cholesterol and fatty acids; Tonz et al., 1975; Land et al., 1988). In vitro studies with SnPP demonstrated that its photophysical parameters (high quantum yield and long triplet lifetime) and singlet oxygen-sensitizing ability are similar to metal-free porphyrins, and it was thus expected to have phototoxic effects in vivo (Land et al., 1988). The triplet lifetime of SnMP has been found to be much higher than SnPP; the addition of quenching groups, like iodine, to the macrocycle reduced the triplet lifetime [tin diiododeuteroporphyrin (SnI2DP)] (Fort and Gold, 1989). As expected, also the excitation wavelengths influenced the triplet lifetimes of these Mps (Delaney et al., 1988). In vivo, all three compounds caused photosensitization in guinea pigs, with SnPP being the strongest photosensitizer and, SnI2DP and SnMP having less photoreactivity probably due to the higher potency and thus use at lower doses for SnMP and the quenching iodine for SnI2DP (Fort and Gold, 1989). In general, it appears that the photophysical properties found in vitro do not completely translate to in vivo conditions. In a different study, mortality was detected in rats treated with SnPP and SnMP and simultaneous exposure to cool white fluorescent light, with an LD50 of 11.7 μmol/kg BW for SnPP and 40% mortality for SnMP at a dose of 20 μmol/kg BW (Hintz et al., 1990). No mortality was observed in rats exposed to similar light conditions after treatment with ZnPP and ZnMP. In human subjects, transient erythema have been reported following treatment with SnPP and SnMP (Kappas et al., 1988, 1995; Berglund et al., 1990; Galbraith et al., 1992; Valaes et al., 1994). The underlying mechanisms, which lead to lethality in the rats after SnPP or SnMP treatment and light exposure are not known. Interestingly, toxicity has also been reported in a study with rhesus monkeys given 25 and 100 μmol SnPP/kg BW. The study was not designed to investigate photosensitizing effects, and information about the quality of light exposure is not given. Nonetheless, death associated with light exposure is conceivable at these high doses, especially, since biopsies revealed cutaneous bullae and dermal inflammation. Moreover, gross histology of livers, spleens, and kidneys showed evidence of infarction (Cornelius and Rodgers, 1984).
In recent studies by our laboratory, we observed phototoxic effects of ZnBG in neonatal mice and found a significant increase in lipid peroxidation in liver and heart tissues after intraperitoneal (i.p.) administration of 30 μmol/kg BW and light exposure. This was accompanied by elevations in aspartate aminotransferase (AST) and creatine kinase activities, inferring the possibility of heart and liver damage (Schulz et al., 2012). We also established that the LD50 for ZnBG was 19.5 μmol/kg BW, which is similar to an LD50 of 23 μmol/kg BW shown in earlier studies in rats (Vreman et al., 1991). In general, ZnPP and ZnMP appear to be far less photoreactive than the tin derivatives in vitro (Vreman and Stevenson, 1990; Vreman et al., 1990a, 1993) and with no phototoxicity in vivo at concentrations up to 60 and 45 μmol/kg BW, respectively (Hintz et al., 1990). Also, the chromium derivatives are not photoreactive in vitro (Vreman and Stevenson, 1990; Vreman et al., 1990a, 1993), and we have recently found that CrMP showed no phototoxicity in vivo. However, we did observe a chemical toxicity with CrMP (Schulz et al., 2012), which is in agreement with a previous study by Lutton et al. (1997), who showed that CrMP given at a dose of 10 μmol/kg was lethal in rabbits. In summary, the in vivo phototoxicity potential of the studied Mps appears to follow this pattern: SnPP > SnMP ≥ ZnBG > ZnMP > ZnPP. Moreover, these studies indicate that the degree of photodamage caused by Mps can be influenced by several factors, including the dose, route of administration, state of Mp aggregation, the time between administration and light exposure, and the spectral quality of the light.
Other side effects
Due to the blockade of heme metabolism, Mps subsequently reduce the CO and free iron status of cells. This, as well as their heme analog structure, may affect hemoproteins and other enzymes. Several studies demonstrated that SnPP diminishes CYP450 capacity and, thus reduces corticosterone levels, CYP450-related drug metabolism, and the CYP450 content in testes (Stout and Becker, 1988; Maines and Trakshel, 1992b; Trakshel et al., 1992). Others showed that hepatic CYP450 content is only transiently altered after administration of SnPP or SnMP to neonatal rats and does not persist into adulthood. The studies used several different doses and application routes to adult or neonatal rats (Drummond et al., 1989, 1996). Overall, SnPP and SnMP decrease CYP450 activity and thus affect CYP450-dependent enzymes of adrenal synthesis and drug metabolism in animal models. However, clinical studies with SnPP and SnMP lack information about these parameters (see Clinical Studies). Although the zinc derivatives appear to not affect the hepatic CYP450 system (Trakshel et al., 1992), inhibition of hematopoiesis by ZnPP and ZnMP was found in vitro in animal and human bone marrow (Lutton et al., 1997). ZnMP, but not SnMP, also displayed inhibitory action on hematopoiesis and on mobilization of progenitor cells in vivo (Lutton et al., 1999). The underlying mechanisms are still unclear, and might not exclusively be attributed to the type of central metal (Zn), but also to the side chains of the porphyrin ring, because ZnBG did not affect bone marrow cell growth (Lutton et al., 1991). Most Mps seem to interact with NOS and sGC, but to different degrees. CrMP and ZnBG have been shown to marginally impair the activity of NOS and sGC at concentrations that effectively inhibit HO activity, and thus seem to be more selective toward HO than ZnPP and SnPP (Appleton et al., 1999). In a different study, SnMP was also found to have minimal effects on hippocampal NOS activity similar to that of ZnBG (Meffert et al., 1994). Recently, it has been shown that CrMP (also SnPP and SnMP) negatively affects systemic macro hemodynamics and the hepatic microcirculation. Intravenous administration of 40 μmol CrMP/kg BW to rats decreased mean arterial pressure, sinusoidal diameter, and hepatic blood flow, and induced hemolysis, marked inflammatory responses, and increased AST levels. SnMP displayed the least effects on those parameters in this study compared to SnPP and CrMP (Scheingraber et al., 2009). The described side effects of CrMP could be responsible, at least in part, for its toxicity seen in certain animal models (see Photosensitivity) and thus its use in human neonates should be discouraged.
Iron deficiency anemia has been reported following long-term treatment with SnMP in rats (Boni et al., 1993) and patients with Crigler–Najjar Syndrome Type I (110 doses of SnMP 0.5 or 1.0 μmol/kg BW during a 400-day study; Boni et al., 1993; Kappas et al., 1993). Because SnMP inhibits intestinal HO (Vreman et al., 1989) and decreases intestinal heme–iron absorption (Boni et al., 1993), this may account for the iron deficiency-like anemia that results after long-term SnMP exposure. Kappas et al. (1993) reported that the deficiency was easily reversed by supplementation with iron. It is also interesting to speculate that Mps may be useful clinically in the treatment of iron overload.
Administration of SnPP or SnMP transiently increased the acute phase protein ferritin, in healthy volunteers as well as in patients with primary biliary cirrhosis or idiopathic hemochromatosis (Berglund et al., 1999). The underlying mechanism is unclear, particularly, since it would be expected that, due to the release of free iron, ferritin levels would increase in response to HO activation, but not due to inhibition (Figure 1).
Of interest is also the possible passage of Mps through the blood–brain barrier and the subsequent effects in this tissue. A study by Drummond and Kappas (1986) showed that SnPP given s.c. crossed placenta and blood–brain barrier of neonatal rats and subsequently inhibited brain HO activity. The blood–brain barrier is most permeable immediately after birth up to a period between 20 and 28 days of postnatal life, suggesting that the ability of SnPP to enter the brain is age-dependent. However, its clearance t1/2 was 1.7 days and therefore relatively rapid compared to other tissues (Drummond and Kappas, 1986). Studies with adult rats also observed low but detectable levels of SnPP in the brain, which are cleared relatively rapidly (Anderson et al., 1984). Intravenous administration of SnPP to adult rats markedly decreased HO and NADPH–CYP450 reductase activity in the brain (Mark and Maines, 1992). In contrast ZnPP, SnMP, CrMP, ZnMP did not appear to affect brain HO activity after i.v. (ZnPP) or s.c. administration to adult rats (Mark and Maines, 1992; Bundock et al., 1996). In the adult brain, HO-2 is the isoenzyme predominantly expressed. In contrast, HO-1 expression in the brain is developmentally regulated, being highest in the early gestational ages and progressively decreasing during the perinatal period to adulthood (Zhao et al., 2006). The constitutive HO-2 isoform is important in the maintenance of neuronal function, whereas HO-1 is believed to play a protective role (Snyder et al., 1998; Maines, 2000). Therefore, it is conceivable that HO inhibition in the brain is not desired, although some have speculated that it may be advantageous in premature infants with intracranial bleeding, where a possibility of enhanced local bilirubin formation exists (Drummond and Kappas, 1986). In studies using hippocampal slices, CrMP, SnMP, ZnPP, and ZnBG all inhibited HO, however only CrMP and ZnPP reduced long-term potentiation (LTP) and also inhibited NOS, which is speculated to be the underlying mechanism for LTP reduction (Meffert et al., 1994).
HO-1 promoter activation
HO-1 gene expression is induced by its substrate heme and a variety of stimuli, e.g., heat shock, oxidative stress, hyperoxia, hypoxia, heavy metals, ultraviolet A radiation, pro-inflammatory mediators, Mps, and many others (Ryter et al., 2006).
Using our HO-1-luc transgenic mouse model where the transgene contains the full-length HO-1 promoter driving expression of the reporter gene luciferase (luc), we found increased reporter gene expression after SnMP and ZnPP treatment, which depended on the route of application, differing from 3-fold to 10-fold (Zhang et al., 2002). Further studies in mice confirmed that the SnMP-mediated induction of the HO-1 gene subsequently leads to a significant increase in HO-1 protein (Morioka et al., 2006). Clinical studies do not report about induced HO-1 protein expression, but describe sufficient reductions of TB levels without a rebound. Therefore it is conceivable that the induction of HO-1 is negligible in the doses used in human studies, which are at least one to two orders of magnitude less than those used animal studies (see Clinical Studies), reinforcing the observation that care must be taken when extrapolating animal studies to the human circumstance.
Several regulatory elements have been shown to be crucial for the activation of the HO-1 gene in response to different stimuli. Bach1, a leucine zipper protein, is a transcriptional repressor. Upon exposure to heme, Bach1 dissociates from its heterodimerization partners within the distal enhancer of the HO-1 promoter and is exported out of the nucleus. Displacement of Bach1 leads to recruitment of the activating NF-E2-related factor 2 (Nrf2) and thus, stimulates HO-1 gene expression (Abate et al., 2007). Bonkovsky and co-workers have demonstrated that CoPP, and ZnMP upregulate HO-1 expression through the repression of Bach1 and upregulation of the Nrf2 protein (Shan et al., 2006; Hou et al., 2008). Our laboratory has shown that SnMP not only induces HO-1 expression by binding to Bach1, but also by increasing Bach1 protein degradation, and thereby affecting the HO-1 promoter directly and indirectly, respectively (Abate et al., 2007).
ZnBG appears to be less effective in HO-1 upregulation, only producing small changes in HO-1 transcription and protein in newborn and adult mice given a heme load (Morioka et al., 2006; He et al., 2011). This induction of HO-1 by ZnBG might be an indirect effect due to the accumulation of heme after inhibition of HO enzyme activity and not a direct interaction with Bach1 (unpublished data).
Pharmacokinetics of Selected Metalloporphyrins
Due to effects of the central metal ion and especially to the lipophilicity or hydrophilicity of the side chains, Mps differ in their pharmacokinetic properties, stability, and solubility. Because the protoporphyrin derivatives are the most lipophilic, their solubility in aqueous solution is minimal. The meso derivatives share similar chemical properties. However, the incorporation of the two bis glycol side chains to the porphyrin ring renders the molecule more polar, thus increasing its solubility in aqueous solutions. In general, all Mps are highly soluble and stable in alkaline aqueous solutions or basic organic solvents, such as pyridine and ethanolamine (Labbe et al., 1999). Although the pharmacokinetics of SnPP are well-studied, its use was abandoned due to the described side effects (especially phototoxicity). Thus, we will focus on the most promising Mps to date: SnMP, ZnPP, ZnMP, and ZnBG.
SnMP
In general, successful oral administration of Mps would be clinically most desirable. However, the chemical characteristics of most Mps preclude this route of administration. Interestingly, absorptivity appears also to be species-specific. For example, SnMP has been shown to be not orally absorbed by rats (Vreman et al., 1988) and by human subjects (Galbraith and Kappas, 1989), but oral administration of SnMP to adult mice significantly decreased VeCO levels, demonstrating an absorption by the intestine and subsequent systemic effects (Morioka et al., 2006). Others have also shown that SnMP inhibits intestinal HO activity after oral administration (Drummond et al., 1992). Moreover, differences in tissue distribution have been observed between adult and neonatal rats. Tissue concentrations of SnMP given s.c. peaked later in neonatal rats than in adults. In general, SnMP is rapidly cleared from the circulation, but appears to have high tissue “stickiness” (up to 27 days in rats), especially in the liver and spleen, and is also found in the kidney and brain. HO activity was reduced in liver, spleen, kidney (neonates only), and brain (not significant) up to 27 days (spleen), but at different time points after administration and to a greater extent in neonates than in adults (Bundock et al., 1996). A fast plasma clearance following i.v. administration with a plasma half-life of 3.8 h and a log-linear decline (similar to SnPP), was also found in adult healthy volunteers (Galbraith and Kappas, 1989). Moreover, SnMP showed a very low excretion rate in feces and urine, suggesting a rapid uptake into intra- or extravascular spaces and tissue binding (Galbraith and Kappas, 1989). Effective doses in human adults and neonates ranged from 1 to 6 μmol/kg BW (Valaes et al., 1994, 1998; Kappas et al., 1995; Martinez et al., 1999) and in animal studies from 1 to 30 μmol/kg BW (Drummond et al., 1987; Morioka et al., 2006).
ZnPP
ZnPP also needs to be administered parenterally (Vreman et al., 1988). Administration by s.c., i.m., or i.p. have been used frequently in animal studies (Maines, 1981; Qato and Maines, 1985; Rodgers et al., 1996). ZnPP at a dose of 40 μmol/kg BW given s.c. to rhesus neonates reduced TB levels within 24 h and lasted up to 12 days. HO inhibition occurred in the liver and spleen, but not in the kidney or brain (Qato and Maines, 1985; Rodgers et al., 1990). Biliary and urinary excretion also was very low. However, ZnPP is extensively incorporated into RBCs (≈45% of the administered dose; Qato and Maines, 1985). Furthermore, it is endogenously generated in cases of iron deficiency and found located primarily in the RBCs (Labbe et al., 1999). Studies in rats showed that ZnPP is relatively fast-acting (∼4 h after s.c. administration), with a duration of action of 1–4 days after the administration of 40 μmol/kg i.p for hepatic HO inhibition (Hamori et al., 1989). In contrast to the rhesus neonate, concentrations of ZnPP found in the spleen tissues of rats were low, and thus splenic HO inhibition was marginal (Rodgers et al., 1996). The spleen is the site of greatest heme catabolism, and therefore targeted inhibition of splenic HO inhibition could increase the in vivo effectiveness of Mps in reducing TB levels. This approach has been attempted through incorporating Mps into liposomes. This strategy significantly increased Mp delivery to the spleen and thus enhanced their efficacy (Landaw et al., 1989; Cannon et al., 1993; Hamori et al., 1993).
ZnMP
Interestingly, ZnMP binds very tightly to human serum albumin (Greenbaum and Kappas, 1991), thus its tissue accessibility is actually very low (27%; Bundock et al., 1996). Therefore, it did not significantly inhibit HO activity in any tissue after s.c. injection (rat) with 1–10 μmol/kg BW. A similar dose range of SnMP significantly reduced HO activity in liver and spleen rat tissue up to 4 and 27 days, respectively. In contrast, when 15-μmol ZnMP/kg BW, bound to albumin in a 1:1 ratio, was administered i.v., it was rapidly cleared from plasma (half-life = 3.6 h), with uptake occurring primarily in liver and spleen (less in the kidney), but was not detected in brain (rats). Inhibition of liver HO activity was still 50% 1 week after administration (Russo et al., 1995).
ZnBG
ZnBG has a higher hydrophilicity due to the bis glycol side chains. It is, besides CrMP, the only Mp, proven to be orally absorbed by mice and rats (Vallier et al., 1991a,b; Morioka et al., 2006). ZnBG is absorbed relatively quickly (within 15 min; Vallier et al., 1991a), highly effective toward inhibiting spleen and liver HO activities, has a rapid onset of action (≈70% inhibition after 1–3 h of administration) and is cleared by the kidneys in 2-week-old suckling rats (Vallier et al., 1991b). In adult rats, HO inhibition after an oral dose of 30 μmol ZnBG/kg BW was approximately 20% (liver), 50% (spleen), and 0% (intestine) after 48 h compared to 60% (liver), 80% (spleen), and 40% (intestine) inhibition with the same dose of SnMP, demonstrating a short duration of action for ZnBG compared to other Mps (Morioka et al., 2006). Supporting data were conducted in a hemolytic mouse model with 1-week-old mice, measuring the bilirubin production as VeCO. The bilirubin production returned back to baseline 6 h after oral gavage of 15 μmol ZnBG/kg BW (He et al., 2011). After i.p. injection to 3-day-old mice of very low doses of ZnBG (0.325 μmol/kg/BW) HO inhibition was 50% after 3 h and returned to baseline after 24 h (Katayama et al., 2012). Negligible amounts of ZnBG (<0.001% of the administered dose) have been found in the brain after oral administration to neonatal rats (Vallier et al., 1991b). No inhibition of HO in the brain of mouse neonates was found after oral gavage of up to 30 μmol ZnBG/kg/BW 3 h after administration, which let us conclude that ZnBG does not pass the blood–brain barrier (He et al., 2011). However, after i.p. administration of 3.75–15 μmol ZnBG/kg BW to 3-day-old mice we observed 30–45% HO inhibition in the brain 3 h after administration (unpublished data). If this discrepancy between both studies may be due to the route of administration, or the fact that the blood–brain barrier is more permeable to many chemicals in the immediate postnatal period (Drummond and Kappas, 1986), but possible not permeable to ZnBG anymore in the 1-week-old mice, needs further investigation.
Summary and Conclusion
Although Mps have been studied extensively in animal models and some human trials, their safety has not been unequivocally proven yet. Ideally, a desirable Mp should have high potency and selectivity toward inhibiting HO without affecting other enzymes, not be photosensitizing, not alter HO-1 gene expression and protein levels, be short-acting, be easily eliminated without the subsequent release of the sequestered metal or preferably contain an essential metal atom, and be orally absorbable (Vreman et al., 2001; Table 2).
ZnBG appears to have many of these desirable pharmacologic and pharmacokinetic properties, and thus appears to be a promising anti-hyperbilirubinemia drug. Its advantages due to in vitro and in vivo animal studies include: its extremely high potency, oral absorptivity, short duration of action, no long-term deposition in tissues, minimal interference with hemoproteins, and minimal effects on HO-1 gene expression and subsequent protein synthesis. Even though ZnBG is photoreactive and shows phototoxicity after i.p. administration, those effects appear negligible when administered orally and in therapeutic doses (≤7.5 μmol/kg BW established in newborn mice) due to its high potency and short duration of action, which minimizes the time neonates need to be protected from direct light exposure. Moreover, a short duration of action would allow the pediatrician in a clinical setting to better “titrate” more accurately the dose required to lower TB levels without the danger of its accumulation in certain tissues and thus minimizing long-term side effects.
Currently pediatricians are dependent upon one frontline treatment strategy: phototherapy, which is well established, successful, and generally safe, at least for larger infants. However, using CO detection technologies and antenatal analyses of genetic predispositions to identify infants at high risk for developing hyperbilirubinemia, could enable us to seek and treat high producers of the pigment, in particular those with hemolysis, who might benefit most from targeted Mp treatment. Introduced in this strategic way, Mps still represent a promising alternative in the management of neonatal jaundice, although more work is required to define safe preventive or therapeutic approaches.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Mary L. Johnson Research Fund, the Christopher Hess Research Fund, and the H.M. Lui Research Fund. Due to space limitations, we would like to apologize to all our colleagues whose research was not cited in this review, but whose work has certainly advanced our understanding in this field.
References
Abate, A., Zhao, H., Wong, R. J., and Stevenson, D. K. (2007). The role of Bach1 in the induction of heme oxygenase by tin mesoporphyrin. Biochem. Biophys. Res. Commun. 354, 757–763.
American Academy of Pediatrics. (2004). Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316.
Anderson, K. E., Simionatto, C. S., Drummond, G. S., and Kappas, A. (1984). Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J. Pharmacol. Exp. Ther. 228, 327–333.
Anderson, K. E., Simionatto, C. S., Drummond, G. S., and Kappas, A. (1986). Disposition of tin-protoporphyrin and suppression of hyperbilirubinemia in humans. Clin. Pharmacol. Ther. 39, 510–520.
Appleton, S. D., Chretien, M. L., McLaughlin, B. E., Vreman, H. J., Stevenson, D. K., Brien, J. F., Nakatsu, K., Maurice, D. H., and Marks, G. S. (1999). Selective inhibition of heme oxygenase, without inhibition of nitric oxide synthase or soluble guanylyl cyclase, by metalloporphyrins at low concentrations. Drug Metab. Dispos. 27, 1214–1219.
Barefield, E. S., Dwyer, M. D., and Cassady, G. (1993). Association of patent ductus arteriosus and phototherapy in infants weighing less than 1000 grams. J. Perinatol. 13, 376–380.
Benders, M. J., Van Bel, F., and Van de Bor, M. (1999). Cardiac output and ductal reopening during phototherapy in preterm infants. Acta Paediatr. 88, 1014–1019.
Berglund, L., Angelin, B., Blomstrand, R., Drummond, G., and Kappas, A. (1988). Sn-protoporphyrin lowers serum bilirubin levels, decreases biliary bilirubin output, enhances biliary heme excretion and potently inhibits hepatic heme oxygenase activity in normal human subjects. Hepatology 8, 625–631.
Berglund, L., Angelin, B., Hultcrantz, R., Einarsson, K., Emtestam, L., Drummond, G., and Kappas, A. (1990). Studies with the haeme oxygenase inhibitor Sn-protoporphyrin in patients with primary biliary cirrhosis and idiopathic haemochromatosis. Gut 31, 899–904.
Berglund, L., Galbraith, R. A., Emtestam, L., Drummond, G. S., Angelin, B., and Kappas, A. (1999). Heme oxygenase inhibitors transiently increase serum ferritin concentrations without altering other acute-phase reactants in man. Pharmacology 59, 51–56.
Boni, R. E., Huch Boni, R. A., Galbraith, R. A., Drummond, G. S., and Kappas, A. (1993). Tin-mesoporphyrin inhibits heme oxygenase activity and heme-iron absorption in the intestine. Pharmacology 47, 318–329.
Bundock, E. A., Drummond, G. S., and Kappas, A. (1996). Tissue distribution of synthetic heme analogues: studies with tin, chromium, and zinc mesoporphyrins. Pharmacology 52, 187–198.
Cannon, J. B., Martin, C., Drummond, G. S., and Kappas, A. (1993). Targeted delivery of a heme oxygenase inhibitor with a lyophilized liposomal tin mesoporphyrin formulation. Pharm. Res. 10, 715–721.
Chernick, R. J., Martasek, P., Levere, R. D., Margreiter, R., and Abraham, N. G. (1989). Sensitivity of human tissue heme oxygenase to a new synthetic metalloporphyrin. Hepatology 10, 365–369.
Cohen, R. S., Wong, R. J., and Stevenson, D. K. (2010). Understanding neonatal jaundice: a perspective on causation. Pediatr. Neonatol. 51, 143–148.
Cornelius, C. E., and Rodgers, P. A. (1984). Prevention of neonatal hyperbilirubinemia in rhesus monkeys by tin-protoporphyrin. Pediatr. Res. 18, 728–730.
Cruse, I., and Maines, M. D. (1988). Evidence suggesting that the two forms of heme oxygenase are products of different genes. J. Biol. Chem. 263, 3348–3353.
Delaney, J. K., Mauzerall, D., Drummond, G. S., and Kappas, A. (1988). Photophysical properties of Sn-porphyrins: potential clinical implications. Pediatrics 81, 498–504.
Dercho, R. A., Nakatsu, K., Wong, R. J., Stevenson, D. K., and Vreman, H. J. (2006). Determination of in vivo carbon monoxide production in laboratory animals via exhaled air. J. Pharmacol. Toxicol. Methods 54, 288–295.
Drummond, G. S., Galbraith, R. A., Sardana, M. K., and Kappas, A. (1987). Reduction of the C2 and C4 vinyl groups of Sn-protoporphyrin to form Sn-mesoporphyrin markedly enhances the ability of the metalloporphyrin to inhibit in vivo heme catabolism. Arch. Biochem. Biophys. 255, 64–74.
Drummond, G. S., and Kappas, A. (1981). Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc. Natl. Acad. Sci. U.S.A. 78, 6466–6470.
Drummond, G. S., and Kappas, A. (1982a). Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model. Science 217, 1250–1252.
Drummond, G. S., and Kappas, A. (1982b). Suppression of hyperbilirubinemia in the rat neonate by chromium-protoporphyrin. Interactions of metalloporphyrins with microsomal heme oxygenase of human spleen. J. Exp. Med. 156, 1878–1883.
Drummond, G. S., and Kappas, A. (1984). An experimental model of postnatal jaundice in the suckling rat. Suppression of induced hyperbilirubinemia by Sn-protoporphyrin. J. Clin. Invest. 74, 142–149.
Drummond, G. S., and Kappas, A. (1986). Sn-protoporphyrin inhibition of fetal and neonatal brain heme oxygenase. Transplacental passage of the metalloporphyrin and prenatal suppression of hyperbilirubinemia in the newborn animal. J. Clin. Invest. 77, 971–976.
Drummond, G. S., Rosenberg, D. W., and Kappas, A. (1992). Intestinal heme oxygenase inhibition and increased biliary iron excretion by metalloporphyrins. Gastroenterology 102, 1170–1175.
Drummond, G. S., Rosenberg, D. W., Kihlstrom-Johanson, A. C., and Kappas, A. (1989). Effects of tin-porphyrins on developmental changes in hepatic cytochrome P450 content, selected P450-dependent drug-metabolizing enzyme activities and brain glutathione levels in the newborn rat. Pharmacology 39, 273–284.
Drummond, G. S., Smith, T. J., and Kappas, A. (1996). Effects of a series of metalloporphyrins on adrenal, testicular and thyroid function in rats. Pharmacology 52, 178–186.
Emtestam, L., Berglund, L., Angelin, B., and Kappas, A. (1989). Treatment of psoriasis vulgaris with a synthetic metalloporphyrin and UVA light. Acta Derm. Venereol. Suppl. (Stockh.) 146, 107–110.
Fort, F. L., and Gold, J. (1989). Phototoxicity of tin protoporphyrin, tin mesoporphyrin, and tin diiododeuteroporphyrin under neonatal phototherapy conditions. Pediatrics 84, 1031–1037.
Galbraith, R. A., Drummond, G. S., and Kappas, A. (1992). Suppression of bilirubin production in the Crigler-Najjar type I syndrome: studies with the heme oxygenase inhibitor tin-mesoporphyrin. Pediatrics 89, 175–182.
Galbraith, R. A., and Kappas, A. (1989). Pharmacokinetics of tin-mesoporphyrin in man and the effects of tin-chelated porphyrins on hyperexcretion of heme pathway precursors in patients with acute inducible porphyria. Hepatology 9, 882–888.
Gathwala, G., and Sharma, S. (2002). Phototherapy induces oxidative stress in premature neonates. Indian J. Gastroenterol. 21, 153–154.
Govaert, P., Lequin, M., Swarte, R., Robben, S., De Coo, R., Weisglas-Kuperus, N., De Rijke, Y., Sinaasappel, M., and Barkovich, J. (2003). Changes in globus pallidus with (pre)term kernicterus. Pediatrics 112, 1256–1263.
Greenbaum, N. L., and Kappas, A. (1991). Comparative photoactivity of tin and zinc porphyrin inhibitors of heme oxygenase: pronounced photolability of the zinc compounds. Photochem. Photobiol. 54, 183–192.
Grundemar, L., and Ny, L. (1997). Pitfalls using metalloporphyrins in carbon monoxide research. Trends Pharmacol. Sci. 18, 193–195.
Hamori, C. J., Lasic, D. D., Vreman, H. J., and Stevenson, D. K. (1993). Targeting zinc protoporphyrin liposomes to the spleen using reticuloendothelial blockade with blank liposomes. Pediatr. Res. 34, 1–5.
Hamori, C. J., Vreman, H. J., Rodgers, P. A., and Stevenson, D. K. (1989). Zinc protoporphyrin inhibits CO production in rats. J. Pediatr. Gastroenterol. Nutr. 8, 110–115.
He, C. X., Campbell, C. M., Zhao, H., Kalish, F. S., Schulz, S., Vreman, H. J., Wong, R. J., and Stevenson, D. K. (2011). Effects of zinc deuteroporphyrin bis glycol on newborn mice after heme-loading. Pediatr. Res. 70, 467–472.
Hiles, R. A. (1974). Absorption, distribution and excretion of inorganic tin in rats. Toxicol. Appl. Pharmacol. 27, 366–379.
Hintz, S. R., Kwong, L. K., Vreman, H. J., and Stevenson, D. K. (1987). Recovery of exogenous heme as carbon monoxide and biliary heme in adult rats after tin protoporphyrin treatment. J. Pediatr. Gastroenterol. Nutr. 6, 302–306.
Hintz, S. R., Stevenson, D. K., Yao, Q., Wong, R. J., Das, A., Van Meurs, K. P., Morris, B. H., Tyson, J. E., Oh, W., Poole, W. K., Phelps, D. L., McDavid, G. E., Grisby, C., and Higgins, R. D. (2011). Is phototherapy exposure associated with better or worse outcomes in 501- to 1000-g-birth-weight infants? Acta Paediatr. 100, 960–965.
Hintz, S. R., Vreman, H. J., and Stevenson, D. K. (1990). Mortality of metalloporphyrin-treated neonatal rats after light exposure. Dev. Pharmacol. Ther. 14, 187–192.
Hou, W., Shan, Y., Zheng, J., Lambrecht, R. W., Donohue, S. E., and Bonkovsky, H. L. (2008). Zinc mesoporphyrin induces rapid and marked degradation of the transcription factor Bach1 and up-regulates HO-1. Biochim. Biophys. Acta 1779, 195–203.
Ignarro, L. J., Wood, K. S., and Wolin, M. S. (1984). Regulation of purified soluble guanylate cyclase by porphyrins and metalloporphyrins: a unifying concept. Adv. Cyclic Nucleotide Protein Phosphorylation Res. 17, 267–274.
Kaplan, M., Wong, R. J., Sibley, E., and Stevenson, D. K. (2011). “Neonatal jaundice and liver disease,” in Neonatal-Perinatal Medicine, eds A. A. Fanaroff, R. J. Martin, and M. Walsh (Philadelphia, PA: Mosby, Elsevier Science), 1443–1496.
Kappas, A. (2004). A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics 113, 119–123.
Kappas, A., and Drummond, G. S. (1986). Control of heme metabolism with synthetic metalloporphyrins. J. Clin. Invest. 77, 335–339.
Kappas, A., Drummond, G. S., and Galbraith, R. A. (1993). Prolonged clinical use of a heme oxygenase inhibitor: hematological evidence for an inducible but reversible iron-deficiency state. Pediatrics 91, 537–539.
Kappas, A., Drummond, G. S., Henschke, C., and Valaes, T. (1995). Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics 95, 468–474.
Kappas, A., Drummond, G. S., Manola, T., Petmezaki, S., and Valaes, T. (1988). Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics 81, 485–497.
Kappas, A., Drummond, G. S., Munson, D. P., and Marshall, J. R. (2001a). Sn-Mesoporphyrin interdiction of severe hyperbilirubinemia in Jehovah’s witness newborns as an alternative to exchange transfusion. Pediatrics 108, 1374–1377.
Kappas, A., Drummond, G. S., and Valaes, T. (2001b). A single dose of Sn-mesoporphyrin prevents development of severe hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns. Pediatrics 108, 25–30.
Kappas, A., Drummond, G. S., Simionatto, C. S., and Anderson, K. E. (1984). Control of heme oxygenase and plasma levels of bilirubin by a synthetic heme analogue, tin-protoporphyrin. Hepatology 4, 336–341.
Kappas, A., Simionatto, C. S., Drummond, G. S., Sassa, S., and Anderson, K. E. (1985). The liver excretes large amounts of heme into bile when heme oxygenase is inhibited competitively by Sn-protoporphyrin. Proc. Natl. Acad. Sci. U.S.A. 82, 896–900.
Katayama, Y., Kalish, F. S., Zhao, H., Wong, R. J., and Stevenson, D. K. (2012). Effect of a low dose of zinc deuteroporphyrin bis glycol in inhibiting heme oxygenase activity after heme-loading. J. Invest. Med. 60, 210–301.
Labbe, R. F., Vreman, H. J., and Stevenson, D. K. (1999). Zinc protoporphyrin: a metabolite with a mission. Clin. Chem. 45, 2060–2072.
Land, E. J., McDonagh, A. F., McGarvey, D. J., and Truscott, T. G. (1988). Photophysical studies of tin(IV)-protoporphyrin: potential phototoxicity of a chemotherapeutic agent proposed for the prevention of neonatal jaundice. Proc. Natl. Acad. Sci. U.S.A. 85, 5249–5253.
Landaw, S. A., Drummond, G. S., and Kappas, A. (1989). Targeting of heme oxygenase inhibitors to the spleen markedly increases their ability to diminish bilirubin production. Pediatrics 84, 1091–1096.
Luo, D., and Vincent, S. R. (1994). Metalloporphyrins inhibit nitric oxide-dependent cGMP formation in vivo. Eur. J. Pharmacol. 267, 263–267.
Lutton, J. D., Abraham, N. G., Drummond, G. S., Levere, R. D., and Kappas, A. (1997). Zinc porphyrins: potent inhibitors of hematopoieses in animal and human bone marrow. Proc. Natl. Acad. Sci. U.S.A. 94, 1432–1436.
Lutton, J. D., Chertkov, J. L., Levere, R. D., and Abraham, N. G. (1991). Comparative effect of heme analogues on hematopoiesis in lymphoproliferative disorders. Leuk. Lymphoma 5, 179–185.
Lutton, J. D., Jiang, S., Drummond, G. S., Abraham, N. G., and Kappas, A. (1999). Comparative pharmacology of zinc mesoporphyrin and tin mesoporphyrin: toxic actions of zinc mesoporphyrin on hematopoiesis and progenitor cell mobilization. Pharmacology 58, 44–50.
Maines, M. D. (1981). Zinc. Protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim. Biophys. Acta 673, 339–350.
Maines, M. D. (2000). The heme oxygenase system and its functions in the brain. Cell. Mol. Biol. (Noisy-le-grand) 46, 573–585.
Maines, M. D., and Trakshel, G. M. (1992a). Differential regulation of heme oxygenase isozymes by Sn- and Zn-protoporphyrins: possible relevance to suppression of hyperbilirubinemia. Biochim. Biophys. Acta 1131, 166–174.
Maines, M. D., and Trakshel, G. M. (1992b). Tin-protoporphyrin: a potent inhibitor of hemoprotein-dependent steroidogenesis in rat adrenals and testes. J. Pharmacol. Exp. Ther. 260, 909–916.
Maines, M. D., Trakshel, G. M., and Kutty, R. K. (1986). Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 261, 411–419.
Maisels, M. J., and McDonagh, A. F. (2008). Phototherapy for neonatal jaundice. N. Engl. J. Med. 358, 920–928.
Mark, J. A., and Maines, M. D. (1992). Tin-protoporphyrin-mediated disruption in vivo of heme oxygenase-2 protein integrity and activity in rat brain. Pediatr. Res. 32, 324–329.
Martasek, P., Solangi, K., Goodman, A. I., Levere, R. D., Chernick, R. J., and Abraham, N. G. (1988). Properties of human kidney heme oxygenase: inhibition by synthetic heme analogues and metalloporphyrins. Biochem. Biophys. Res. Commun. 157, 480–487.
Martinez, J. C., Garcia, H. O., Otheguy, L. E., Drummond, G. S., and Kappas, A. (1999). Control of severe hyperbilirubinemia in full-term newborns with the inhibitor of bilirubin production Sn-mesoporphyrin. Pediatrics 103, 1–5.
Maurer, H. M., Kirkpatrick, B. V., McWilliams, N. B., Draper, D. A., and Bryla, D. A. (1985). Phototherapy for hyperbilirubinemia of hemolytic disease of the newborn. Pediatrics 75, 407–412.
McCoubrey, W. K. Jr., Huang, T. J., and Maines, M. D. (1997). Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 247, 725–732.
McCoubrey, W. K. Jr., and Maines, M. D. (1994). The structure, organization and differential expression of the gene encoding rat heme oxygenase-2. Gene 139, 155–161.
McDonagh, A. F., and Palma, L. A. (1985). Tin-protoporphyrin: a potent photosensitizer of bilirubin destruction. Photochem. Photobiol. 42, 261–264.
McMillan, J. J., Vreman, H. J., and Stevenson, D. K. (1987). Tin-protoporphyrin inhibits carbon monoxide production in adult male Wistar rats with common bile duct ligation. J. Pediatr. Gastroenterol. Nutr. 6, 795–798.
Meffert, M. K., Haley, J. E., Schuman, E. M., Schulman, H., and Madison, D. V. (1994). Inhibition of hippocampal heme oxygenase, nitric oxide synthase, and long-term potentiation by metalloporphyrins. Neuron 13, 1225–1233.
Morioka, I., Wong, R. J., Abate, A., Vreman, H. J., Contag, C. H., and Stevenson, D. K. (2006). Systemic effects of orally-administered zinc and tin (IV) metalloporphyrins on heme oxygenase expression in mice. Pediatr. Res. 59, 667–672.
Morris, B. H., Oh, W., Tyson, J. E., Stevenson, D. K., Phelps, D. L., O’Shea, T. M., McDavid, G. E., Perritt, R. L., Van Meurs, K. P., Vohr, B. R., Grisby, C., Yao, Q., Pedroza, C., Das, A., Poole, W. K., Carlo, W. A., Duara, S., Laptook, A. R., Salhab, W. A., Shankaran, S., Poindexter, B. B., Fanaroff, A. A., Walsh, M. C., Rasmussen, M. R., Stoll, B. J., Cotten, C. M., Donovan, E. F., Ehrenkranz, R. A., Guillet, R., and Higgins, R. D. (2008). Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N. Engl. J. Med. 359, 1885–1896.
Penn, A. A., Enzmann, D. R., Hahn, J. S., and Stevenson, D. K. (1994). Kernicterus in a full term infant. Pediatrics 93, 1003–1006.
Qato, M. K., and Maines, M. D. (1985). Prevention of neonatal hyperbilirubinaemia in non-human primates by Zn-protoporphyrin. Biochem. J. 226, 51–57.
Reddy, P., Najundaswamy, S., Mehta, R., Petrova, A., and Hegyi, T. (2003). Tin-mesoporphyrin in the treatment of severe hyperbilirubinemia in a very-low-birth-weight infant. J. Perinatol. 23, 507–508.
Rodgers, P. A., Seidman, D. S., Wei, P. L., Dennery, P. A., and Stevenson, D. K. (1996). Duration of action and tissue distribution of zinc protoporphyrin in neonatal rats. Pediatr. Res. 39, 1041–1049.
Rodgers, P. A., Vreman, H. J., and Stevenson, D. K. (1990). Heme catabolism in rhesus neonates inhibited by zinc protoporphyrin. Dev. Pharmacol. Ther. 14, 216–222.
Russo, S. M., Pepe, J. A., Donohue, S., Cable, E. E., Lambrecht, R. W., and Bonkovsky, H. L. (1995). Tissue distribution of zinc-mesoporphyrin in rats: relationship to inhibition of heme oxygenase. J. Pharmacol. Exp. Ther. 272, 766–774.
Ryter, S. W., Alam, J., and Choi, A. M. (2006). Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86, 583–650.
Sassa, S., Drummond, G. S., Bernstein, S. E., and Kappas, A. (1983). Tin-protoporphyrin suppression of hyperbilirubinemia in mutant mice with severe hemolytic anemia. Blood 61, 1011–1013.
Scheidt, P. C., Bryla, D. A., and Hoffman, H. J. (1987). Phototherapy and patent ductus arteriosus. Pediatrics 80, 593–594.
Scheingraber, S., Messner, S., Matt, S., Abel, K., Goger, S., Kotner, K., Schilling, M. K., and Menger, M. D. (2009). Metalloporphyrins, used for HO-1 inhibition, themselves affect hepatic microcirculation, liver function, and hepatocellular integrity. Microcirculation 16, 355–363.
Schuller, D. J., Wilks, A., Ortiz de Montellano, P. R., and Poulos, T. L. (1999). Crystal structure of human heme oxygenase-1. Nat. Struct. Biol. 6, 860–867.
Schulz, S., Wong, R. J., Kalish, F. S., Zhao, H., Jang, K. Y., Vreman, H. J., and Stevenson, D. K. (2012). The effect of light exposure on metalloporphyrin-treated newborn mice. Pediatr. Res. (in press).
Shan, Y., Lambrecht, R. W., Donohue, S. E., and Bonkovsky, H. L. (2006). Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 20, 2651–2653.
Snyder, S. H., Jaffrey, S. R., and Zakhary, R. (1998). Nitric oxide and carbon monoxide: parallel roles as neural messengers. Brain Res. Brain Res. Rev. 26, 167–175.
Stevenson, D. K., Bartoletti, A. L., Ostrander, C. R., and Johnson, J. D. (1979). Pulmonary excretion of carbon monoxide in the human newborn infant as an index of bilirubin production: III. Measurement of pulmonary excretion of carbon monoxide after the first postnatal week in premature infants. Pediatrics 64, 598–600.
Stevenson, D. K., Rodgers, P. A., and Vreman, H. J. (1989). The use of metalloporphyrins for the chemoprevention of neonatal jaundice. Am. J. Dis. Child. 143, 353–356.
Stevenson, D. K., Vreman, H. J., Oh, W., Fanaroff, A. A., Wright, L. L., Lemons, J. A., Verter, J., Shankaran, S., Tyson, J. E., Korones, S. B., Bauer, C. B., Stoll, B. J., Papile, L.-A., Okah, F., and Ehrenkranz, R. A. (1994). Bilirubin production in healthy term infants as measured by carbon monoxide in breath. Clin. Chem. 40, 1934–1939.
Stevenson, D. K., Vreman, H. J., and Wong, R. J. (2011). Bilirubin production and the risk of bilirubin neurotoxicity. Semin. Perinatol. 35, 121–126.
Stevenson, D. K., and Wong, R. J. (2010). Metalloporphyrins in the management of neonatal hyperbilirubinemia. Semin. Fetal Neonatal Med. 15, 164–168.
Stout, D. L., and Becker, F. F. (1988). The effects of tin-protoporphyrin administration on hepatic xenobiotic metabolizing enzymes in the juvenile rat. Drug Metab. Dispos. 16, 23–26.
Suresh, G. K., Martin, C. L., and Soll, R. F. (2003). Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst. Rev. 2, CD004207.
Tenhunen, R., Marver, H. S., and Schmid, R. (1968). The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. U.S.A. 61, 748–755.
Tenhunen, R., Ross, M. E., Marver, H. S., and Schmid, R. (1970). Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry 9, 298–303.
Tonz, O., Vogt, J., Filippini, L., Simmler, F., Wachsmuth, E. D., and Winterhalter, K. H. (1975). Severe light dermatosis following photo therapy in a newborn infant with congenital erythropoietic urophyria. Helv. Paediatr. Acta 30, 47–56.
Trakshel, G. M., Kutty, R. K., and Maines, M. D. (1986). Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J. Biol. Chem. 261, 11131–11137.
Trakshel, G. M., Kutty, R. K., and Maines, M. D. (1988). Resolution of the rat brain heme oxygenase activity: absence of a detectable amount of the inducible form (HO-1). Arch. Biochem. Biophys. 260, 732–739.
Trakshel, G. M., Sluss, P. M., and Maines, M. D. (1992). Comparative effects of tin- and zinc-protoporphyrin on steroidogenesis: tin-protoporphyrin is a potent inhibitor of cytochrome P-450-dependent activities in the rat adrenals. Pediatr. Res. 31, 196–201.
Travadi, J., Simmer, K., Ramsay, J., Doherty, D., and Hagan, R. (2006). Patent ductus arteriosus in extremely preterm infants receiving phototherapy: does shielding the chest make a difference? A randomized, controlled trial. Acta Paediatr. 95, 1418–1423.
Valaes, T., Drummond, G. S., and Kappas, A. (1998). Control of hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns using an inhibitor of bilirubin production, Sn-mesoporphyrin. Pediatrics 101, E1.
Valaes, T., Petmezaki, S., Henschke, C., Drummond, G. S., and Kappas, A. (1994). Control of jaundice in preterm newborns by an inhibitor of bilirubin production: studies with tin-mesoporphyrin. Pediatrics 93, 1–11.
Vallier, H. A., Rodgers, P. A., Castillo, R. O., and Stevenson, D. K. (1991a). Absorption of zinc deuteroporphyrin IX 2,4-bis-glycol by the neonatal rat small intestine in vivo. Dev. Pharmacol. Ther. 17, 109–115.
Vallier, H. A., Rodgers, P. A., and Stevenson, D. K. (1991b). Oral administration of zinc deuteroporphyrin IX 2,4 bis glycol inhibits heme oxygenase in neonatal rats. Dev. Pharmacol. Ther. 17, 220–222.
Vreman, H. J., Baxter, L. M., Stone, R. T., and Stevenson, D. K. (1996). Evaluation of a fully automated end-tidal carbon monoxide instrument for breath analysis. Clin. Chem. 42, 50–56.
Vreman, H. J., Ekstrand, B. C., and Stevenson, D. K. (1993). Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr. Res. 33, 195–200.
Vreman, H. J., Gillman, M. J., Downum, K. R., and Stevenson, D. K. (1990a). In vitro generation of carbon monoxide from organic molecules and synthetic metalloporphyrins mediated by light. Dev. Pharmacol. Ther. 15, 112–124.
Vreman, H. J., Rodgers, P. A., and Stevenson, D. K. (1990b). Zinc protoporphyrin administration for suppression of increased bilirubin production by iatrogenic hemolysis in rhesus neonates. J. Pediatr. 117, 292–297.
Vreman, H. J., Gillman, M. J., and Stevenson, D. K. (1989). In vitro inhibition of adult rat intestinal heme oxygenase by metalloporphyrins. Pediatr. Res. 26, 362–365.
Vreman, H. J., Hintz, S. R., Kim, C. B., Castillo, R. O., and Stevenson, D. K. (1988). Effects of oral administration of tin and zinc protoporphyrin on neonatal and adult rat tissue heme oxygenase activity. J. Pediatr. Gastroenterol. Nutr. 7, 902–906.
Vreman, H. J., Lee, O. K., and Stevenson, D. K. (1991). In vitro and in vivo characteristics of a heme oxygenase inhibitor: ZnBG. Am. J. Med. Sci. 302, 335–341.
Vreman, H. J., and Stevenson, D. K. (1990). Metalloporphyrin-enhanced photodegradation of bilirubin in vitro. Am. J. Dis. Child. 144, 590–594.
Vreman, H. J., Stevenson, D. K., Oh, W., Fanaroff, A. A., Wright, L. L., Lemons, J. A., Wright, E., Shankaran, S., Tyson, J. E., Korones, S. B., Bauer, C. R., Stoll, B. J., Papile, L.-A., Donovan, E. F., and Ehrenkranz, R. A. (1994). Semiportable electrochemical instrument for determining carbon monoxide in breath. Clin. Chem. 40, 1927–1933.
Vreman, H. J., Wong, R. J., Harmatz, P., Fanaroff, A. A., Berman, B., and Stevenson, D. K. (1999). Validation of the natus CO-stat end tidal breath analyzer in children and adults. J. Clin. Monit. Comput. 15, 421–427.
Vreman, H. J., Wong, R. J., Murdock, J. R., and Stevenson, D. K. (2008). Standardized bench method for evaluating the efficacy of phototherapy devices. Acta Paediatr. 97, 308–316.
Vreman, H. J., Wong, R. J., and Stevenson, D. K. (2001). Alternative metalloporphyrins for the treatment of neonatal jaundice. J. Perinatol. 21(Suppl. 1), S108–S113.
Vreman, H. J., Wong, R. J., and Stevenson, D. K. (2004). Phototherapy: current methods and future directions. Semin. Perinatol. 28, 326–333.
Wong, R. J., Bhutani, V. K., Vreman, H. J., and Stevenson, D. K. (2007). Tin mesoporphyrin for the prevention of severe neonatal hyperbilirubinemia. Pharmacol. Rev. 8, e77–e84.
Wong, R. J., Vreman, H. J., Schulz, S., Kalish, F. S., Pierce, N. W., and Stevenson, D. K. (2011). In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J. Perinatol. 31(Suppl. 1), S35–S41.
Yoshinaga, T., Sassa, S., and Kappas, A. (1982). Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J. Biol. Chem. 257, 7778–7785.
Zhang, W., Contag, P. R., Hardy, J., Zhao, H., Vreman, H. J., Hajdena-Dawson, M., Wong, R. J., Stevenson, D. K., and Contag, C. H. (2002). Selection of potential therapeutics based on in vivo spatiotemporal transcription patterns of heme oxygenase-1. J. Mol. Med. 80, 655–664.
Keywords: bilirubin, heme oxygenase, hemolysis, neonatal hyperbilirubinemia
Citation: Schulz S, Wong RJ, Vreman HJ and Stevenson DK (2012) Metalloporphyrins – an update. Front. Pharmacol. 3:68. doi: 10.3389/fphar.2012.00068
Received: 02 February 2012; Accepted: 03 April 2012;
Published online: 26 April 2012.
Edited by:
Mahin D. Maines, University of Rochester School of Medicine, USAReviewed by:
Thor Willy Ruud Hansen, Oslo University Hospital, NorwayJon Watchko, University of Pittsburgh School of Medicine, USA
Copyright: © 2012 Schulz, Wong, Vreman and Stevenson. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Ronald J. Wong, Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine, 300 Pasteur Drive, Room S230, Stanford, CA 94305-5208, USA. e-mail: rjwong@stanford.edu
 Stephanie Schulz
Stephanie Schulz