Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment
Abstract
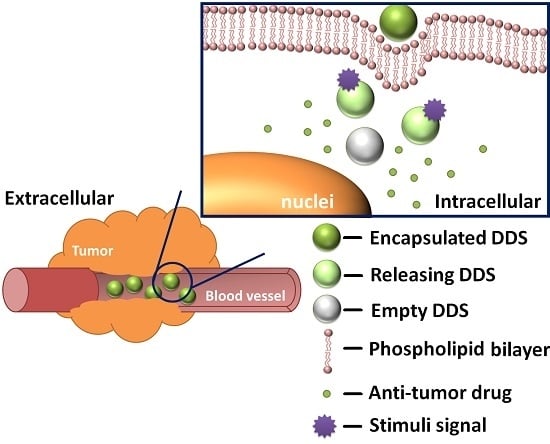
:1. Introduction
2. Major DDS Scaffolds
2.1. Mesoporous Nanoparticles
2.2. Polymers
2.3. Metal-Organic Frameworks
2.4. Quantum Dots
2.5. Carbon Nanotubes
3. Stimuli-Responsive Mechanisms
3.1. Intrinsic Stimuli
3.1.1. pH-Responsiveness
- Acid-caused charge reversal;
- Acid-caused dissolution behavior change;
- Acid-caused hydrolysis reactions and bond breaking.
3.1.2. Redox-Responsiveness
3.1.3. Biomolecule-Responsiveness
3.2. Extrinsic Stimuli
3.2.1. Thermo-Responsiveness
3.2.2. Light-Responsiveness
3.2.3. Magnetic Field Responsiveness
3.2.4. Ultrasound Responsiveness
4. Clinical Development
5. Major Challenges and Outlook
- Protein corona effect of nanocarriers. Inside the human body, during the blood transport process, blood proteins have a tendency to cover the surface of DDS through hydrophobic, van der Waals or electrostatic interactions [159]. This phenomenon generally results in enhanced non-specific cellular uptake and has not been intensively studied until recently. The interaction between proteins and nanoparticles is closely related to the physical and chemical properties of the nanomaterial itself. Particle size, charge distribution and other parameters could affect the binding between DDS and proteins. According to the related researches, for silica nanoparticles, polystyrene (PS) nanospheres, and Au nanoparticles, their affinity to protein weakens with decreasing size, but it does not appear to be a universal conclusion; nanoparticle shape also plays a role in protein combination. Serum proteins are mainly negatively charged, therefore, to minimize the serum protein adsorption, DDSs with electronegative surfaces would be better. According to the report of Muller and coworkers, hydrophobic surfaces are prone to be covered by protein substances compared to hydrophilic surfaces. To avoid the protein corona effect, researchers have done systematic work to augment the stealth capability of DDS. PEGylation is a common strategy for prolonging the circulation time and reduce non-specific protein adsorption. Recently, poly(phosphoester)s (PPEs) have been proposed as an effective alternative for PEG.
- Developing DDS for combination drug therapy. With rapid advancement of cancer treatment studies, single small molecule cancer drugs are no longer the only solution for chemotherapy. Combination drug therapy has been proved to show significantly better outcomes than single drug treatments by suppressing drug resistance and synergistic effects. Recently, interference genes and growth factors have gradually become hot research topics for effective cancer treatment. Together with anti-cancer drugs, this new combination therapy opens up new possibilities for enhanced anticarcinogenic bioactivity [160]. However, current DDSs are limited to the single drug delivery level; few of them have achieved multi-drug co-delivery results. As we have seen from Table 1 and Table 2, the majority of the conceptual DDSs only adopted DOX as model drug. The key problems that hinder successful construction of co-delivery DDSs include: unclear anticancer mechanisms of dual-drugs and precise control of the drug release order.
- Imperfect tumor targeting effects. Compared with stimuli-responsive drug delivery, site specific drug delivery is an equally important function of an effective DDS. Targeting functions could be built through two mechanisms: passive targeting and active targeting. For passive targeting mode, the key rationale is based on the EPR effect. As the DDS platform becomes diversified, superparamagnetism has been increasingly applied with the introduction of magnetic nanoparticles. Therefore, the location of DDSs inside the human body could be precisely controlled via an external magnetic field. Magnetic orientation has been developed as an effective passive targeting mechanism in recent studies [139,157]. However, passive targeting could not remarkably improve the intracellular uptake of DDS. This inherent limitation has promoted more effective targeting mechanisms. Active targeting mode endows the DDS with “smart judgement” to discriminate the cancer cells. Recent researches revealed that some specific kinds of receptors such as folate and transferrin are overexpressed in cancer cells. Thus bioconjugation of antibodies of these receptors on the surface of nanoscale DDSs can actively deliver DDS into cancer cells. Even though targeted ligands can result in improved cellular uptake, few of them could be clinically applied. This is because the surface properties of the DDSs will be profoundly affected with the conjugation of active targeting ligand and the changes brought by active targeting ligand have yet to be fully understood.
- Lack of multistimuli-responsive functions. After a DDS enters the human body, we expect it to respond in real time to an efficient stimulus signal and hence, adjust to our demand. However, for single stimulus-responsive DDSs, the drug release process is very likely to be disturbed by extensive interference factors. Due to the heterogeneity of tumor tissues, the pathological characteristics in different kinds of tumors or in different stages of a single tumor are quite diverse. Intelligent DDS, requires an accurate response in a very narrow window. To address this problem, researchers have developed multi-stimuli-responsive mechanisms for building DDSs with high performance. The combination of two or more stimuli-responsive groups are usually parallel or causal. Even more selectivity can be achieved if these responsive groups could be designed to be serial or to work in a Boolean logic pattern. Zink et al. developed an analyte-responsive gate [161]. All stimuli signals were firstly “analyzed” by the smart DDS. The release behavior would only be triggered if two specific stimuli existed simultaneously. In other words, the two specific values are true in this AND gate. If this logic gate could be applied in DDS, stimuli-responsive drug release could be more precisely controlled.
- Biodegradation problems. After the drug release process, the biodegradation of the DDS becomes an additional problem. As foreign substances can induce an immune response, probably disturbing the anti-tumor drug mechanism. For an ideal, biodegradable DDS, the carrier subject should remain intact. The disassembly process should not take place until the beginning or end of the release behavior. The degraded substances should meet all bio-safety standards and can be easily discharged through metabolism.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Reichman, B.; Hakes, T.; Curtin, J.; Jones, W.; Lewis, J.L.; Barakat, R.; Rubin, S.; Mychalczak, B.; Saigo, P.; et al. Intraperitoneal chemotherapy in the management of ovarian cancer. Cancer 1993, 71, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Oesterreich, S.; Weng, C.-N.; Qiu, M.; Hilsenbeck, S.G.; Osborne, C.K.; Fuqua, S.A.W. The Small Heat Shock Protein hsp27 Is Correlated with Growth and Drug Resistance in Human Breast Cancer Cell Lines. Cancer Res. 1993, 53, 4443–4448. [Google Scholar] [PubMed]
- Lévi, F.; Misset, J.-L.; Brienza, S.; Adam, R.; Metzger, G.; Itzakhi, M.; Caussanel, J.-P.; Kunstlinger, F.; Lecouturier, S.; Descorps-Declère, A.; et al. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer 1992, 69, 893–900. [Google Scholar] [CrossRef]
- Waters, J.S.; Norman, A.; Cunningham, D.; Scarffe, J.H.; Webb, A.; Harper, P.; Joffe, J.K.; Mackean, M.; Mansi, J.; Leahy, M.; et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: Results of a randomized trial. Br. J. Cancer 1999, 80, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Ohe, Y.; Ohashi, Y.; Kubota, K.; Tamura, T.; Nakagawa, K.; Negoro, S.; Nishiwaki, Y.; Saijo, N.; Ariyoshi, Y.; Fukuoka, M. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann. Oncol. 2007, 18, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, Z.; Zhao, Y. Hybrid Nanoparticles as Drug Carriers for Controlled Chemotherapy of Cancer. Chem. Rec. 2016, 16, 1833–1851. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, J. Hollow-Structured Mesoporous Materials: Chemical Synthesis, Functionalization and Applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, S.A.; Schmidt, A.; Weiss, V.; Argyo, C.; von Schirnding, C.; Bein, T.; Bräuchle, C. Targeted Drug Delivery in Cancer Cells with Red-Light Photoactivated Mesoporous Silica Nanoparticles. Nano Lett. 2013, 13, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.; Kwok, A.; Fang, W.; Ma, X.; Nguyen, K.T.; Korzh, V.; Zhao, Y. Functional Mesoporous Silica Nanoparticles for Photothermal-Controlled Drug Delivery in Vivo. Angew. Chem. Int. Ed. 2012, 51, 8373–8377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, K.; Liu, J.; Sun, G.; Yin, Q.; Lu, L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials 2015, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Li, P.-Z.; Nguyen, K.T.; Wang, X.-J.; Luo, Z.; Zhang, H.; Tan, N.S.; Zhao, Y. Biocompatible, Uniform, and Redispersible Mesoporous Silica Nanoparticles for Cancer-Targeted Drug Delivery In Vivo. Adv. Funct. Mater. 2014, 24, 2450–2461. [Google Scholar] [CrossRef]
- Zhang, P.; Cheng, F.; Zhou, R.; Cao, J.; Li, J.; Burda, C.; Min, Q.; Zhu, J.-J. DNA-Hybrid-Gated Multifunctional Mesoporous Silica Nanocarriers for Dual-Targeted and MicroRNA-Responsive Controlled Drug Delivery. Angew. Chem. Int. Ed. 2014, 53, 2371–2375. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; West, K.R.; Scherman, O.A. Hollow mesoporous raspberry-like colloids with removable caps as photoresponsive nanocontainers. Nanoscale 2016, 8, 7840–7844. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.D.; Thamm, D.H.; Kraft, S.L.; Boyes, S.G. Polymer-Modified Gadolinium Metal-Organic Framework Nanoparticles Used as Multifunctional Nanomedicines for the Targeted Imaging and Treatment of Cancer. Biomacromolecules 2009, 10, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-L.; Song, N.; Zhang, S.X.-A.; Li, H.; Wang, B.; Yang, Y.-W. Ca2+, pH and thermo triple-responsive mechanized Zr-based MOFs for on-command drug release in bone diseases. J. Mater. Chem. B 2016, 4, 135–140. [Google Scholar] [CrossRef]
- Tan, L.-L.; Li, H.; Zhou, Y.; Zhang, Y.; Feng, X.; Wang, B.; Yang, Y.-W. Zn2+-Triggered Drug Release from Biocompatible Zirconium MOFs Equipped with Supramolecular Gates. Small 2015, 11, 3807–3813. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-Organic Frameworks: A Rapidly Growing Class of Versatile Nanoporous Materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug. Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ye, E.; David; Lakshminarayanan, R.; Loh, X.J. Recent Advances of Using Hybrid Nanocarriers in Remotely Controlled Therapeutic Delivery. Small 2016. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Wang, Y.; Wang, X.; Wang, Q.; Chen, M. Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol. Adv. 2014, 32, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.-F.; Xu, X.-D.; Zhang, J.; Yang, J.; Gong, Y.-H.; Lei, Q.; Jia, H.-Z.; Li, C.; Zhuo, R.-X.; Zhang, X.-Z. Encapsulation of an Adamantane-Doxorubicin Prodrug in pH-Responsive Polysaccharide Capsules for Controlled Release. ACS Appl. Mater. Interfaces 2012, 4, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Hou, C.; Ren, J.; Xin, X.; Pei, Y.; Lu, Y.; Cao, S.; Pei, Z. Multifunctional supramolecular vesicles based on the complex of ferrocene carboxylic acid capped pillar[5]arene and a galactose derivative for targeted drug delivery. Chem. Commun. 2016, 52, 9578–9581. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gong, G.; Feng, J.; Wang, T.; Ding, C.; Zhou, B.; Jiang, W.; Fu, J. Dual pH-Mediated Mechanized Hollow Zirconia Nanospheres. ACS Appl. Mater. Interface 2016, 8, 23289–23301. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-J.; Liang, H.-F.; Chen, H.-L.; Wang, Y.; Cheng, P.-Y.; Liu, H.-L.; Xia, Y.; Sung, H.-W. A Thermoresponsive Bubble-Generating Liposomal System for Triggering Localized Extracellular Drug Delivery. ACS Nano 2013, 7, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart multifunctional magnetic nanoparticle-based drug delivery system for cancer thermo-chemotherapy and intracellular imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ren, J.; Li, J.; Leng, J.; Qu, Y.; Lin, C.; Shi, D. Magnetothermally responsive star-block copolymeric micelles for controlled drug delivery and enhanced thermo-chemotherapy. Nanoscale 2015, 7, 9655–9663. [Google Scholar] [CrossRef] [PubMed]
- Kakwere, H.; Leal, M.P.; Materia, M.E.; Curcio, A.; Guardia, P.; Niculaes, D.; Marotta, R.; Falqui, A.; Pellegrino, T. Functionalization of Strongly Interacting Magnetic Nanocubes with (Thermo)Responsive Coating and Their Application in Hyperthermia and Heat-Triggered Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 10132–10145. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Devi, K.S.P.; Banerjee, R.; Maiti, T.K.; Pramanik, P.; Dhara, D. Thermal and pH Responsive Polymer-Tethered Multifunctional Magnetic Nanoparticles for Targeted Delivery of Anticancer Drug. ACS Appl. Mater. Interfaces 2013, 5, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive Intelligent Drug Delivery Systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, A.; Guardia, P.; Curcio, A.; Garcia, M.A.; Cingolani, R.; Manna, L.; Pellegrino, T. Subnanometer Local Temperature Probing and Remotely Controlled Drug Release Based on Azo-Functionalized Iron Oxide Nanoparticles. Nano Lett. 2013, 13, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Poelma, S.O.; Oh, S.S.; Helmy, S.; Knight, A.S.; Burnett, G.L.; Soh, H.T.; Hawker, C.J.; Read de Alaniz, J. Controlled drug release to cancer cells from modular one-photon visible light-responsive micellar system. Chem. Commun. 2016, 52, 10525–10528. [Google Scholar] [CrossRef] [PubMed]
- Rwei, A.Y.; Wang, W.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Hu, J.-J.; Xu, Q.; Chen, S.; Jia, H.-Z.; Sun, Y.-X.; Zhuo, R.-X.; Zhang, X.-Z. A redox-responsive drug delivery system based on RGD containing peptide-capped mesoporous silica nanoparticles. J. Mater. Chem. B 2015, 3, 39–44. [Google Scholar] [CrossRef]
- Jin, S.; Wan, J.; Meng, L.; Huang, X.; Guo, J.; Liu, L.; Wang, C. Biodegradation and Toxicity of Protease/Redox/pH Stimuli-Responsive PEGlated PMAA Nanohydrogels for Targeting Drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 19843–19852. [Google Scholar] [CrossRef] [PubMed]
- Noyhouzer, T.; L’Homme, C.; Beaulieu, I.; Mazurkiewicz, S.; Kuss, S.; Kraatz, H.-B.; Canesi, S.; Mauzeroll, J. Ferrocene-Modified Phospholipid: An Innovative Precursor for Redox-Triggered Drug Delivery Vesicles Selective to Cancer Cells. Langmuir 2016, 32, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Gadde, S. Multi-drug delivery nanocarriers for combination therapy. Med. Chem. Commun. 2015, 6, 1916–1929. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; He, W.; Zhao, D.; Song, A.; Luan, Y. Disulfide-Linked Amphiphilic Polymer-Docetaxel Conjugates Assembled Redox-Sensitive Micelles for Efficient Antitumor Drug Delivery. Biomacromolecules 2016, 17, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Xia, Y. A Reactive Oxygen Species (ROS)-Responsive Polymer for Safe, Efficient, and Targeted Gene Delivery in Cancer Cells. Angew. Chem. Int. Ed. 2013, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. High-Frequency, Magnetic-Field-Responsive Drug Release from Magnetic Nanoparticle/Organic Hybrid Based on Hyperthermic Effect. ACS Appl. Mater. Interfaces 2010, 2, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Maquieira, A.; Amorós, P. Controlled Delivery Using Oligonucleotide-Capped Mesoporous Silica Nanoparticles. Angew. Chem. 2010, 122, 7439–7441. [Google Scholar] [CrossRef]
- Zhong, J.; Li, L.; Zhu, X.; Guan, S.; Yang, Q.; Zhou, Z.; Zhang, Z.; Huang, Y. A smart polymeric platform for multistage nucleus-targeted anticancer drug delivery. Biomaterials 2015, 65, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Hao, N.; Chen, H.-Y.; Xu, J.-J. Tumor-Marker-Mediated “on-Demand” Drug Release and Real-Time Monitoring System Based on Multifunctional Mesoporous Silica Nanoparticles. Anal. Chem. 2014, 86, 10239–10245. [Google Scholar] [CrossRef] [PubMed]
- Tukappa, A.; Ultimo, A.; de la Torre, C.; Pardo, T.; Sancenón, F.; Martínez-Máñez, R. Polyglutamic Acid-Gated Mesoporous Silica Nanoparticles for Enzyme-Controlled Drug Delivery. Langmuir 2016, 32, 8507–8515. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Mekaru, H.; Lu, J.; Tamanoi, F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015, 95, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shen, H.; Mao, J.; Zhang, L.; Jiang, Z.; Sun, T.; Lan, Q.; Zhang, Z. Transferrin Modified Graphene Oxide for Glioma-Targeted Drug Delivery: In Vitro and in Vivo Evaluations. ACS Appl. Mater. Interfaces 2013, 5, 6909–6914. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tan, A.; Prestidge, C.A.; Nielsen, H.M.; Müllertz, A. Self-nanoemulsifying drug delivery systems for oral insulin delivery: In vitro and in vivo evaluations of enteric coating and drug loading. Int. J. Pharm. 2014, 477, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.A.; Lu, J.; Tamanoi, F.; Zink, J.I. Functional Nanovalves on Protein-Coated Nanoparticles for In vitro and In vivo Controlled Drug Delivery. Small 2015, 11, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Hu, Y.; Cai, K.; Ding, X.; Zhang, Q.; Li, M.; Ma, X.; Zhang, B.; Zeng, Y.; Li, P.; et al. Intracellular redox-activated anticancer drug delivery by functionalized hollow mesoporous silica nanoreservoirs with tumor specificity. Biomaterials 2014, 35, 7951–7962. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Andreani, T.; Fangueiro, J.; Faggio, C.; Silva, C.; Santini, A.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 2015, 70, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Dullaart, A.; Bock, A.-K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Saha, B.; Ghosh, S.K.; Pradeep Singh, N.D. Photoresponsive quinoline tethered fluorescent carbon dots for regulated anticancer drug delivery. Chem. Commun. 2013, 49, 10471–10473. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-G.; Xia, Y.-Y. Electrochemical capacitance characterization of NiO with ordered mesoporous structure synthesized by template SBA-15. Electrochim. Acta 2006, 51, 3223–3227. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.Y. Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release. Accounts. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, W.H.J.; Diniz da Costa, J.C.; Drennan, J.; Lu, G.Q. Proton conductivity of mesoporous sol-gel zirconium phosphates for fuel cell applications. J. Mater. Chem. 2005, 15, 754–758. [Google Scholar] [CrossRef]
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissortel, F.; Salbeck, J.; Spreitzer, H.; Gratzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar]
- Antonelli, D.M.; Ying, J.Y. Synthesis of a Stable Hexagonally Packed Mesoporous Niobium Oxide Molecular Sieve through a Novel Ligand-Assisted Templating Mechanism. Angew. Chem. Int. Ed. 1996, 35, 426–430. [Google Scholar] [CrossRef]
- Luo, J.-y.; Wang, Y.-g.; Xiong, H.-m.; Xia, Y.-y. Ordered Mesoporous Spinel LiMn2O4 by a Soft-Chemical Process as a Cathode Material for Lithium-Ion Batteries. Chem. Mater. 2007, 19, 4791–4795. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, S.; Hyeon, T.; Oh, S.M.; Bum Kim, K. Synthesis of a new mesoporous carbon and its application to electrochemical double-layer capacitors. Chem. Commun. 1999, 21, 2177–2178. [Google Scholar] [CrossRef]
- Lin, C.T.; Lee, S.Y.; Keh, E.S.; Dong, D.R.; Huang, H.M.; Shih, Y.H. Influence of silanization and filler fraction on aged dental composites. J. Oral Rehabil. 2000, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Panwar, N.; Tng, D.J.H.; Tjin, S.C.; Wang, K.; Yong, K.-T. The application of mesoporous silica nanoparticle family in cancer theranostics. Coord. Chem. Rev. 2016, 319, 86–109. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Li, X.; Liu, X.; Yang, Q. Organosilane-Assisted Transformation from Core-Shell to Yolk-Shell Nanocomposites. Chem. Mater. 2011, 23, 3676–3684. [Google Scholar] [CrossRef]
- Okamoto, M.; Tsukada, H.; Fukasawa, S.; Sakajiri, A. Synthesis of hollow and rattle-type mesoporous silica spheres by treating layered mesoporous silica with a basic solution, and using the spheres as microreactors for two-phase reactions. J. Mater. Chem. A 2015, 3, 11880–11890. [Google Scholar] [CrossRef]
- Deng, Y.; Qi, D.; Deng, C.; Zhang, X.; Zhao, D. Superparamagnetic High-Magnetization Microspheres with an Fe3O4@SiO2 Core and Perpendicularly Aligned Mesoporous SiO2 Shell for Removal of Microcystins. J. Am. Chem. Soc. 2008, 130, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Zhang, C.; Sun, W.; Liu, H.; Jin, Y.; Li, Z.; Liang, X.-J.; Jia, G.; Zhang, J. Up-Conversion Y2O3:Yb3+,Er3+ Hollow Spherical Drug Carrier with Improved Degradability for Cancer Treatment. ACS Appl. Mater. Interfaces 2016, 8, 25078–25086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Li, Z.; Kabehie, S.; Botros, Y.Y.; Stoddart, J.F.; Zink, J.I. pH-Operated Nanopistons on the Surfaces of Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2010, 132, 13016–13025. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, M.; Ding, C.; Fu, J. Mono-benzimidazole functionalized β-cyclodextrins as supramolecular nanovalves for pH-triggered release of p-coumaric acid. Chem. Commun. 2014, 50, 12469–12472. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Liu, Y.; Wang, T.; Fu, J. Triple-stimuli-responsive nanocontainers assembled by water-soluble pillar[5]arene-based pseudorotaxanes for controlled release. J. Mater. Chem. B 2016, 4, 2819–2827. [Google Scholar] [CrossRef]
- Zhang, Y.; Ang, C.Y.; Li, M.; Tan, S.Y.; Qu, Q.; Luo, Z.; Zhao, Y. Polymer-Coated Hollow Mesoporous Silica Nanoparticles for Triple-Responsive Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 18179–18187. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Xu, S.-H.; Zhou, H.; Wang, X.; Dong, B.; Gao, H.; Tang, J.; Yang, Y.-W. pH and Glutathione Dual-Responsive Dynamic Cross-Linked Supramolecular Network on Mesoporous Silica Nanoparticles for Controlled Anticancer Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 28656–28664. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Allen, S.; Kang, Y.; Katie, V.; Liao, Y.; Chang, C.; Timothy, D.; Andre, N.; Meng, H. Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano 2016, 10, 2702–2715. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Trewyn, B.G.; Stellmaker, M.P.; Lin, V.S.Y. Stimuli-Responsive Controlled-Release Delivery System Based on Mesoporous Silica Nanorods Capped with Magnetic Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, X.; Du, X. pH- and redox-triggered synergistic controlled release of a ZnO-gated hollow mesoporous silica drug delivery system. J. Mater. Chem. B 2015, 3, 1426–1432. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.-Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.Y. A Polyamidoamine Dendrimer-Capped Mesoporous Silica Nanosphere-Based Gene Transfection Reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, Y.; Lin, V.S.Y.; Slowing, I.I.; Trewyn, B.G. Luciferase and Luciferin Co-immobilized Mesoporous Silica Nanoparticle Materials for Intracellular Biocatalysis. J. Am. Chem. Soc. 2011, 133, 18554–18557. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, G.; Wang, M.; Zhou, B.; Fu, J. Voltage/pH-Driven Mechanized Silica Nanoparticles for the Multimodal Controlled Release of Drugs. ACS Appl. Mater. Interfaces 2015, 7, 21295–21304. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Dong, H.; Cai, X.; Wang, D.; Li, Y. Mesoporous Silica Nanoparticles Capped with Disulfide-Linked PEG Gatekeepers for Glutathione-Mediated Controlled Release. ACS Appl. Mater. Interfaces 2012, 4, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Choi, E.S.; Cheon, J.Y.; Joo, S.H.; Ryu, J.-H. Noncovalent Polymer-Gatekeeper in Mesoporous Silica Nanoparticles as a Targeted Drug Delivery Platform. Adv. Funct. Mater. 2015, 25, 957–965. [Google Scholar] [CrossRef]
- He, D.; He, X.; Wang, K.; Cao, J.; Zhao, Y. A Light-Responsive Reversible Molecule-Gated System Using Thymine-Modified Mesoporous Silica Nanoparticles. Langmuir 2012, 28, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Sun, Y.; Sun, Y.-L.; Wen, J.; Zhou, Y.; Bing, Q.-M.; Isaacs, L.D.; Jin, Y.; Gao, H.; Yang, Y.-W. Mesoporous Silica Nanoparticles Coated by Layer-by-Layer Self-assembly Using Cucurbit[7]uril for in Vitro and in Vivo Anticancer Drug Release. Chem. Mater. 2014, 26, 6418–6431. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, X.; Ashley, C.; Brinker, C.J. Electrostatically Mediated Liposome Fusion and Lipid Exchange with a Nanoparticle-Supported Bilayer for Control of Surface Charge, Drug Containment, and Delivery. J. Am. Chem. Soc. 2009, 131, 7567–7569. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, J.; Wang, X.; Yu, Y.; Zhang, H.; Chen, Y.; Liu, J.; Sun, Z.; Zou, H.; Sun, D.; et al. The eradication of breast cancer cells and stem cells by 8-hydroxyquinoline-loaded hyaluronan modified mesoporous silica nanoparticle-supported lipid bilayers containing docetaxel. Biomaterials 2013, 34, 7662–7673. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Epler, K.E.; Padilla, D.P.; Phillips, G.K.; Castillo, R.E.; Wilkinson, D.C.; Wilkinson, B.S.; Burgard, C.A.; Kalinich, R.M.; et al. Delivery of Small Interfering RNA by Peptide-Targeted Mesoporous Silica Nanoparticle-Supported Lipid Bilayers. ACS Nano 2012, 6, 2174–2188. [Google Scholar] [CrossRef] [PubMed]
- Epler, K.; Padilla, D.; Phillips, G.; Crowder, P.; Castillo, R.; Wilkinson, D.; Wilkinson, B.; Burgard, C.; Kalinich, R.; Townson, J.; et al. Delivery of Ricin Toxin A-Chain by Peptide-Targeted Mesoporous Silica Nanoparticle-Supported Lipid Bilayers. Adv. Healthc. Mater. 2012, 1, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Desai, D.; Rosenholm, J.M. Tethered Lipid Bilayer Gates: Toward Extended Retention of Hydrophilic Cargo in Porous Nanocarriers. Adv. Funct. Mater. 2014, 24, 2352–2360. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, L.; Song, W.; Wu, Z.; Li, D. Biocompatible polymer materials: Role of protein-surface interactions. Prog. Polym. Sci. 2008, 33, 1059–1087. [Google Scholar] [CrossRef]
- Maciel, D.; Figueira, P.; Xiao, S.; Hu, D.; Shi, X.; Rodrigues, J.; Tomás, H.; Li, Y. Redox-Responsive Alginate Nanogels with Enhanced Anticancer Cytotoxicity. Biomacromolecules 2013, 14, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Johnston, A.P.R.; Dodds, S.J.; Kamphuis, M.M.J.; Ferguson, C.; Parton, R.G.; Nice, E.C.; Heath, J.K.; Caruso, F. Uptake and Intracellular Fate of Disulfide-Bonded Polymer Hydrogel Capsules for Doxorubicin Delivery to Colorectal Cancer Cells. ACS Nano 2010, 4, 2928–2936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Xiao, A.; Yu, H. The synthesis of modified polyethylene via coordination polymerization followed by ATRP, RAFT, NMRP or ROP. Prog. Polym. Sci. 2010, 35, 1195–1216. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.; Yang, W.; Cui, L.; Zhang, J.; Liu, J. Graphene/tri-block copolymer composites prepared via RAFT polymerizations for dual controlled drug delivery via pH stimulation and biodegradation. Eur. Polym. J. 2015, 69, 559–572. [Google Scholar] [CrossRef]
- Zhang, Q.; Re Ko, N.; Kwon Oh, J. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Ghandehari, H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shi, X. Dendrimer-based nanodevices for targeted drug delivery applications. J. Mater. Chem. B 2013, 1, 4199–4211. [Google Scholar] [CrossRef]
- Ye, F.; Barrefelt, Å.; Asem, H.; Abedi-Valugerdi, M.; El-Serafi, I.; Saghafian, M.; Abu-Salah, K.; Alrokayan, S.; Muhammed, M.; Hassan, M. Biodegradable polymeric vesicles containing magnetic nanoparticles, quantum dots and anticancer drugs for drug delivery and imaging. Biomaterials 2014, 35, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Mukai, S.-A.; Sawada, S.-I.; Sasaki, Y.; Akiyoshi, K. Nanocarrier-Integrated Microspheres: Nanogel Tectonic Engineering for Advanced Drug-Delivery Systems. Adv. Mater. 2015, 27, 5080–5088. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ma, X.; Zhang, B.; Zhou, Z.; Jin, E.; Shen, Y.; van Kirk, E.A.; Murdoch, W.J.; Radosz, M.; Sun, W. Fabrication of dendrimer-releasing lipidic nanoassembly for cancer drug delivery. Biomater. Sci. 2016, 4, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, L.; Ying, X.; Jian, Y.; Hong, Y.; Hu, F.; Du, Y. Antitumor Drug Delivery Modulated by a Polymeric Micelle with an Upper Critical Solution Temperature. Angew. Chem. Int. Ed. 2015, 54, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-K.; Jun, S.; Kim, I.; Jin, S.-M.; Kim, J.-G.; Shin, T.J.; Lee, E. Stepwise Drug-Release Behavior of Onion-Like Vesicles Generated from Emulsification-Induced Assembly of Semicrystalline Polymer Amphiphiles. Adv. Funct. Mater. 2015, 25, 4570–4579. [Google Scholar] [CrossRef]
- Alezi, D.; Belmabkhout, Y.; Suyetin, M.; Bhatt, P.M.; Weseliński, Ł.J.; Solovyeva, V.; Adil, K.; Spanopoulos, I.; Trikalitis, P.N.; Emwas, A.-H.; et al. MOF Crystal Chemistry Paving the Way to Gas Storage Needs: Aluminum-Based soc-MOF for CH4, O2, and CO2 Storage. J. Am. Chem. Soc. 2015, 137, 13308–13318. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, P.; Muller, M.; Corma, A. MOF catalysis in relation to their homogeneous counterparts and conventional solid catalysts. Chem. Sci. 2014, 5, 2979–3007. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Enhanced Adsorption of Ammonia on Metal-Organic Framework/Graphite Oxide Composites: Analysis of Surface Interactions. Adv. Funct. Mater. 2010, 20, 111–118. [Google Scholar] [CrossRef]
- Harbuzaru, B.V.; Corma, A.; Rey, F.; Atienzar, P.; Jordá, J.L.; García, H.; Ananias, D.; Carlos, L.D.; Rocha, J. Metal-Organic Nanoporous Structures with Anisotropic Photoluminescence and Magnetic Properties and Their Use as Sensors. Angew. Chem. Int. Ed. 2008, 47, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, A.C.; Xiao, B.; Wragg, D.S.; Wheatley, P.S.; Megson, I.L.; Morris, R.E. Exceptional Behavior over the Whole Adsorption-Storage-Delivery Cycle for NO in Porous Metal Organic Frameworks. J. Am. Chem. Soc. 2008, 130, 10440–10444. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-L.; Li, H.; Qiu, Y.-C.; Chen, D.-X.; Wang, X.; Pan, R.-Y.; Wang, Y.; Zhang, S.X.-A.; Wang, B.; Yang, Y.-W. Stimuli-responsive metal-organic frameworks gated by pillar[5]arene supramolecular switches. Chem. Sci. 2015, 6, 1640–1644. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Takagahara, T.; Takeda, K. Theory of the quantum confinement effect on excitons in quantum dots of indirect-gap materials. Phys. Rev. B 1992, 46, 15578–15581. [Google Scholar] [CrossRef]
- Tang, R.; Xue, J.; Xu, B.; Shen, D.; Sudlow, G.P.; Achilefu, S. Tunable Ultrasmall Visible-to-Extended Near-Infrared Emitting Silver Sulfide Quantum Dots for Integrin-Targeted Cancer Imaging. ACS Nano 2015, 9, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.V.; Nagaoka, Y.; Maekawa, T.; Sakthikumar, D.; Jayasree, R.S. Quantum Dot Tailored to Single Wall Carbon Nanotubes: A Multifunctional Hybrid Nanoconstruct for Cellular Imaging and Targeted Photothermal Therapy. Small 2014, 10, 2771–2775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Xu, Y.-D.; Ma, Y.-Y.; Qiu, L.-L.; Wang, Y.; Kong, J.-L.; Xiong, H.-M. Biodegradable ZnO@polymer Core–Shell Nanocarriers: pH-Triggered Release of Doxorubicin in Vitro. Angew. Chem. Int. Ed. 2013, 52, 4127–4131. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Stewart, M.H.; Trammell, S.A.; Susumu, K.; Delehanty, J.B.; Mei, B.C.; Melinger, J.S.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Quantum-dot/dopamine bioconjugates function as redox coupled assemblies for in vitro and intracellular pH sensing. Nat. Mater. 2010, 9, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Hur, W.; Park, S.-J.; Hong, S.W.; Choi, J.E.; Goh, E.J.; Yoon, S.K.; Hahn, S.K. Bioimaging for Targeted Delivery of Hyaluronic Acid Derivatives to the Livers in Cirrhotic Mice Using Quantum Dots. ACS Nano 2010, 4, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.; Yoong, S.; Anna, J.; Tomasz, P.; Han, K.; Ang, W.; Giorgia, P. Carbon Nanotubes for Delivery of Small Molecule Drugs. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef] [PubMed]
- Bafkary, R.; Khoee, S. Carbon Nanotube-based Stimuli-responsive Nanocarriers for Drug Delivery. RSC Adv. 2016, 6, 82553–82565. [Google Scholar] [CrossRef]
- Takahisa, O.; Yasuhiko, H.; Fumiyoshi, Y.; Mitsuru, H. Development of Novel Drug and Gene Delivery Carriers Composed of Single-Walled Carbon Nanotubes and Designed Peptides with PEGylation. J. Pharm. Sci. 2016, 105, 2815–2824. [Google Scholar]
- Lvye, N.; Meng, L.; Lu, Q. Folate-Conjugated PEG on Single Walled Carbon Nanotubes for Targeting Delivery of Doxorubicin to Cancer Cells. Macromol. Biosci. 2013, 13, 735–744. [Google Scholar]
- Sandeep, V.; Zheng, D.; Giorgia, P.; Khalid, A.; John, L.; Sheu, F. Delivery of drugs and biomolecules using carbon nanotubes. Carbon 2011, 49, 4077–4097. [Google Scholar]
- Kang, B.; Li, J.; Chang, S.; Dai, M.; Ren, C.; Dai, Y.; Chen, D. Subcellular Tracking of Drug Release from Carbon Nanotube Vehicles in Living Cells. Small 2012, 8, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Wang, X.; Wang, X.; Cheng, L.; Li, Y.; Liu, Z. Mesoporous Silica Coated Single-Walled Carbon Nanotubes as a Multifunctional Light-Responsive Platform for Cancer Combination Therapy. Adv. Funct. Mater. 2015, 25, 384–392. [Google Scholar] [CrossRef]
- Li, R.; Wu, R.; Zhao, L.; Hu, Z.; Guo, S.; Pan, X.; Zou, H. Folate and iron difunctionalized multiwall carbon nanotubes as dual-targeted drug nanocarrier to cancer cells. Carbon 2011, 49, 1797–1805. [Google Scholar] [CrossRef]
- Satyajit, D.; Manasmita, D.; Raman, S.; Sanyog, J. Hyaluronate Tethered, “Smart” Multiwalled Carbon Nanotubes for Tumor-Targeted Delivery of Doxorubicin. Bioconjug. Chem. 2012, 23, 2201–2213. [Google Scholar]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-S.; Li, Z.-Y.; Zhu, J.-Y.; Han, K.; Zeng, Z.-Y.; Hong, W.; Li, W.-X.; Jia, H.-Z.; Liu, Y.; Zhuo, R.-X.; et al. Dual-pH Sensitive Charge-Reversal Polypeptide Micelles for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell. Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Lin, C.; Hu, X.-Y.; Wang, L. GSH- and pH-responsive drug delivery system constructed by water-soluble pillar[5]arene and lysine derivative for controllable drug release. Chem. Commun. 2015, 51, 6832–6835. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y.; Li, C.; He, F.; Hou, Z.; Huang, S.; Zhu, H.; Chen, X.; Lin, J. Poly(Acrylic Acid) Modification of Nd3+-Sensitized Upconversion Nanophosphors for Highly Efficient UCL Imaging and pH-Responsive Drug Delivery. Adv. Funct. Mater. 2015, 25, 4717–4729. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, L.; Xu, X.; Bertrand, N.; Choi, W., II; Yameen, B.; Shi, J.; Shah, V.; Mulvale, M.; MacLean, J.L.; et al. Hydrophobic Cysteine Poly(disulfide)-based Redox-Hypersensitive Nanoparticle Platform for Cancer Theranostics. Angew. Chem. Int. Ed. 2015, 54, 9218–9223. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Deng, H.; Su, Y.; He, L.; Wang, R.; Tong, G.; He, D.; Zhu, X. Aptamer-Functionalized and Backbone Redox-Responsive Hyperbranched Polymer for Targeted Drug Delivery in Cancer Therapy. Biomacromolecules 2016, 17, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, F.; Nguyen, K.T.; Ma, X.; Wang, X.; Xing, B.; Zhao, Y. Multifunctional Mesoporous Silica Nanoparticles for Cancer-Targeted and Controlled Drug Delivery. Adv. Funct. Mater. 2012, 22, 5144–5156. [Google Scholar] [CrossRef]
- Zhao, Y.; Berger, R.; Landfester, K.; Crespy, D. Double Redox-Responsive Release of Encoded and Encapsulated Molecules from Patchy Nanocapsules. Small 2015, 11, 2995–2999. [Google Scholar] [CrossRef] [PubMed]
- de Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible Polymeric Nanoparticles Degrade and Release Cargo in Response to Biologically Relevant Levels of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Máñez, R.; Sancenón, F. Gated Materials for on-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, S.; Chen, D.; Li, N.; Li, H.; Xu, Q.; Ge, J.; Lu, J. Light-triggered reversible assemblies of azobenzene-containing amphiphilic copolymer with β-cyclodextrin-modified hollow mesoporous silica nanoparticles for controlled drug release. Chem. Commun. 2012, 48, 10010–10012. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Y.; Jia, K.; Cao, Y.; Li, Y.; Qin, S.; Zhou, F.; Lin, C.; Zhang, D.; Wang, L. Dual Photo- and pH-Responsive Supramolecular Nanocarriers Based on Water-Soluble Pillar[6]arene and Different Azobenzene Derivatives for Intracellular Anticancer Drug Delivery. Chemistry 2015, 21, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Raichur, A.M. Near-infrared light-responsive graphene oxide composite multilayer capsules: A novel route for remote controlled drug delivery. Chem. Commun. 2013, 49, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, P.; Li, X.; Hu, X.; Hou, J.; Wang, L.; Zhang, F. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv. Mater. 2016, 28, 9341–9348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Chen, K.-J.; Noh, S.-H.; Garcia, M.A.; Wang, H.; Lin, W.-Y.; Jeong, H.; Kong, B.J.; Stout, D.B.; Cheon, J.; et al. On-Demand Drug Release System for in Vivo Cancer Treatment through Self-Assembled Magnetic Nanoparticles. Angew. Chem. 2013, 125, 4480–4484. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Schöttler, S.; Landfester, K.; Mailänder, V. Controlling the Stealth Effect of Nanocarriers through Understanding the Protein Corona. Angew. Chem. Int. Ed. 2016, 55, 8806–8815. [Google Scholar] [CrossRef] [PubMed]
- Kolishetti, N.; Dhar, S.; Valencia, P.M.; Lin, L.Q.; Karnik, R.; Lippard, S.J.; Langer, R.; Farokhzad, O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 17939–17944. [Google Scholar]
- Guardado-Alvarez, T.M.; Chen, W.; Norton, A.E.; Russell, M.M.; Connick, W.B..; Zink, J.I. Analyte-responsive gated hollow mesoporous silica nanoparticles exhibiting inverse functionality and an AND logic response. Nanoscale 2016, 8, 18296–18300. [Google Scholar]




| Framework | Functional Factor | Payload | Operation Mechanism | Result | Ref. |
|---|---|---|---|---|---|
| MSNs | Cyclodextrin based supramolecular nanovalve | p-Coumaric Acid | pH-caused dethreading process | Effective pH-triggered release | [84] |
| MSNs | Pillararene based pseudo[2]rotaxane supramolecular nanovalves | DOX | Acid-induced dethreading process | Proliferation inhibition against MCF-7 cells | [85] |
| Hollow MSNs | ZnO quantum dot plug gate | DOX | Dissolution of ZnO in acid environment and cleavage of disulfide linker in GSH environment | Anti-proliferative activity against A549 cancer cells | [91] |
| MSNs | Cyclodextrin based supramolecular nanovalve | DOX & GEM | Acid-induced ketal cleavage and voltage-responsive supramolecular host-guest dedecomplexation | Efficient supression against MCF7 cells | [94] |
| MSNs | PEG polymer gatekeeper | Dye | Cleavage of disulfide linker in GSH environment | Enhanced delivering effect into cancer cells | [95] |
| MSNs | PEG based polymer shell, cyclic (Arg-Gly-Asp-d-Phe-Cys) as targeting ligand | Cisplatin/DOX | GSH-induced cleavage of the wrapped polymer shell | Selective drug delivery to KB cells | [96] |
| MSNs | Dynamic cross-linked supramolecular network of poly(glycidyl methacrylate)s derivative chains | DOX | GSH-induced cleavage of disulfide linker and acid-induced disassembly of the cross-linked polymer network | Good inhibitory effect on A549 cancer cells’ growth | [88] |
| MSNs | Supramolecular bridge gate of CB[7] and bis-aminated poly(glycerolmethacrylate)s | DOX | Acid and competitive binding caused disassembly of supramolecular bridge gate | Efficient stimuli-responsive drug release both in vitro and in vivo | [98] |
| MSNs | Lipid bilayer gate and targeting peptide (SP94) | siRNA | Interruption of electronstatic force between MSNs and lipid bilayer caused by acid stimuli | Repressed gene expression at the protein level and cancer cell apoptosis. | [101] |
| MSNs | Lipid bilayer shell | Calcein | Charge conversion induced cargo release | Successful intracellular delivery of cargo molecules | [103] |
| Dendrimers | Dendrimer/lipid nanoassemblies | DOX | Lipid layer fusion with the cell membrane caused cargo release | Enhanced cellular uptake of DOX | [116] |
| Micelles | Amphiphilic poly(acrylamide-co-acrylonitrile)-g-PEG | DOX | Temperature-related solubility change of micelles | temperaturedependent release of DOX | [117] |
| Vesicles | poly(ethylene oxide)-block-poly(ε-caprolactone) | DOX | Hydrolytic cleavage of caprolactone linker | Prolonging drug retention time, acid stimuli drug release | [118] |
| Hydrogel | Anionic alginate and cystamine | DOX | Cleavage of disulfide linker in GSH environment | Improved in vitro anticancer efficacy against CAL-72 cells | [105] |
| MOFs | Carboxylatopillararene based pseudo[2]rotaxanes gatekeeper | DOX | Acid-induced disassembly of pseudorotaxane gatekeeper | pH-sensitive drug release, negligible intrinsic cytotoxicity | [124] |
| QDs | ZnO@polymer QDs | DOX | Decomposion of ZnO QDs under acid environment | Minimal pre-leakage, controllable drug release within U251 cells, enhanced cell imaging function | [129] |
| QDs | CQDs and quinoline-chlorambucil units | Chlorambucil | Photo-cleavage of quinoline section under UV irradiation | Photoregulated DOX release within Hela cells | [68] |
| CNTs | Chitosan coated SWCNTs and FITC fluorescent label | DOX | Weakened π–π stacking function between DOX and SWCNTs under acid environment | Effective intracellular DOX accumulation inside endothelial progenitor cells | [137] |
| CNTs | Mesoporous coated SWCNTs | DOX | Photothermal heating triggered DOX release under NIR irradiation | Efficient in vivo tumor growth inhibition | [138] |
| CNTs | FA and iron difunctionalized MWCNT | DOX | Photothermal heating triggered DOX release under NIR irradiation and weakened π–π stacking function under acid environment | Enhanced cancer specificity and drug delivery efficiency | [139] |
| CNTs | MWCNT-hyaluronic acid conjugate, Alexa-Fluor-647 fluorescent label | DOX | Weakened π–π stacking function in low pH environment | Selective drug accumulation in A549 cells | [140] |
| Product | Structure | Active Ingredients | Indication | Stimuli | Status |
|---|---|---|---|---|---|
| Doxil® | Pegylated liposome | Doxorubicin | Kaposi’s sarcoma, ovarian cancer and breast cancer | - | Approved by FDA |
| Daunoxome® | Liposome | Daunorubicin | Kaposi’s sarcoma | - | Approved by FDA |
| Caelyx® | Liposome | Doxorubicin | Kaposi’s sarcoma, ovarian cancer and breast cancer | - | Approved by European Union |
| Mepact® | Liposome | Muramyl tripeptide phosphatidyl-ethanolamine | Osteosarcoma | - | Approved by European Union |
| ThermoDox | Liposome | Doxorubicin | Liver cancer and lung cancer | Temperature | Phase III in liver cancer, Phase II in lung cancer |
| Abraxane® | Protein nanoparticle | Paclitaxel | Metastatic breast cancer | - | Approved by FDA |
| Genexol-PM® | Polymeric micelles | Paclitaxel | Breast cancer | - | Approved in Korea |
| SMANCS® | Polymeric conjugate | Neocarzinostatin | Liver cancer | - | Approved in Japan |
| T-DM1® | Antibody conjugate | paclitaxel or docetaxel | Metastatic breast cancer | GSH concentration | Approved by FDA |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Tong, L.; Feng, J.; Fu, J. Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment. Molecules 2016, 21, 1715. https://doi.org/10.3390/molecules21121715
Ding C, Tong L, Feng J, Fu J. Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment. Molecules. 2016; 21(12):1715. https://doi.org/10.3390/molecules21121715
Chicago/Turabian StyleDing, Chendi, Ling Tong, Jing Feng, and Jiajun Fu. 2016. "Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment" Molecules 21, no. 12: 1715. https://doi.org/10.3390/molecules21121715