Published online Jul 21, 2008. doi: 10.3748/wjg.14.4300
Revised: May 1, 2008
Accepted: May 8, 2008
Published online: July 21, 2008
Hepatocellular carcinoma (HCC) is the commonest primary malignant cancer of the liver in the world. Given that the burden of chronic liver disease is expected to rise owing to increasing rates of alcoholism, hepatitis B and C prevalence and obesity-related fatty liver disease, it is expected that the incidence of HCC will also increase in the foreseeable future. This article summarizes the international epidemiology, the risk factors and the pathogenesis of HCC, including the roles of viral hepatitis, toxins, such as alcohol and aflatoxin, and insulin resistance.
- Citation: Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J Gastroenterol 2008; 14(27): 4300-4308
- URL: https://www.wjgnet.com/1007-9327/full/v14/i27/4300.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4300
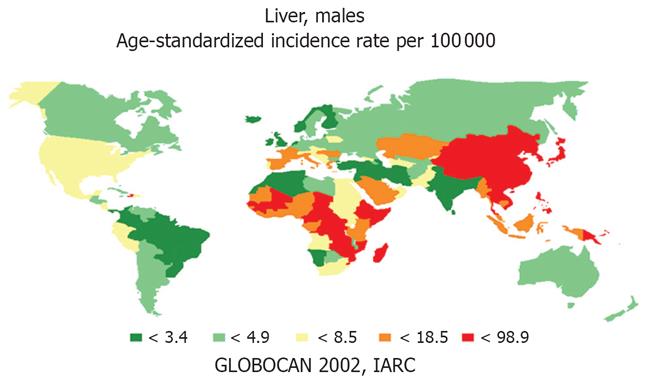
Hepatocellular carcinoma (HCC) is the commonest primary cancer of the liver. Incidence is increasing and HCC has risen to become the 5th commonest malignancy worldwide and the third leading cause of cancer-related death, exceeded only by cancers of the lung and stomach[1]. The estimated incidence of new cases is about 500 000-1 000 000 per year, causing 600 000 deaths globally per year[2–6]. However, important differences have been noted between countries. Most cases of HCC occur in Asia[1] where several countries, particularly in East Asia, have a very high incidence (over 20 cases/100 000 population). For example, the incidence is 99 per 100 000 persons in Mongolia, 49 per 100 000 in Korea, 29 per 100 000 in Japan, and 35 per 100 000 in China[3]. Hong Kong and Thailand also have similarly high rates. Another region of concern is sub-Saharan Africa, particularly the western region of Africa, including Gambia, Guinea, and Mali, and also the Republic of Mozambique in south-east Africa. Areas with moderately high risk (11 cases/100 000-20 cases/100 000) include Italy, Spain and Latin American countries, and those at intermediate risk (5 cases/100 000-10 cases/100 000) include France, the United Kingdom, and the Federal Republic of Germany. A relatively low incidence (less than 5 cases/10 000) is found in the United States, Canada, and in Scandinavia. However, there are large areas of the world where the incidence is still unknown[378].
Although currently relatively low, the incidence of HCC is rising in developed western countries[9–12]. In the United States, there has been an increase of about 80% in the annual incidence of HCC during the past two decades. HCC rates increased from 1.4/100 000 per year from 1976-1980 to 2.4/100 000 per year from 1991-1995[310]. This increase has been most marked in men, with African-American men having higher incidence rates than US Caucasian men. This was explained by the emergence of hepatitis C during this same period, although the rise in immigration from HBV-endemic countries may also have played a role[910].
Other developed western countries have noted similar increasing trends. An increase in incidence of HCC has been reported in Italy, the United Kingdom, Canada, Japan, and Australia. The increase was reported among immigrants from parts of the world with high prevalence, such as sub-Saharan Africa and parts of Asia, being associated with a parallel increase in hospitalization and mortality for HCC[310]. In Egypt, between 1993 and 2002, there was an almost twofold increase in HCC amongst chronic liver patients[13].
However, it is not obvious when this rising trend, observed in many countries, will reach a peak. The Disease Control Center in Atlanta has estimated that deaths related to chronic hepatitis C in the United States will triple from the current rates of 8-10 000 per year during the next decade. While most of these deaths will be due to liver failure and its complications, a considerable proportion can be expected to be due to HCC as well[9].
The most recent World Health report (World Health Organization[14], Table 1) indicated a total of 714 600 new cases of HCC worldwide, with 71% among men (Figure 1). HCC is the 4th commonest cause of death due to cancer, after cancers of the respiratory system, stomach, and colon/rectum. Liver cancer ranked 3rd for male subjects and 5th for women. Geographically, there were 45 000 liver cancer deaths in Africa, 37 000 in the Americas, 15 000 in the eastern Mediterranean, 67 000 in Europe, 61 000 in South-East Asia, and 394 000 in the western Pacific region, including China and Japan. In the same year, 783 000 persons died from cirrhosis, of which 501 000 were men and 282 000 were women[15].
| Year (reference) | Total number | Males | Females |
| 1990 | 437 408 | 316 300 | 121 100 |
| 2000 | 564 300 | 398 364 | 165 972 |
| 2002 (The World health report, 2003) | 714 600 | 504 600 | 210 000 |
The incidence of HCC increases with age, reaching its highest prevalence among those aged over 65 years[1617]. Although HCC is rare before the age of 50 years in North America and Western Europe[18], a shift in incidence towards younger persons has been noted in the last two decades. HCC tends to occur in the background of cirrhosis of the liver. In western countries, this holds true in over 90% of cases, whereas in Asia and Africa the percentage of cases of HCC is higher in individuals with non-cirrhotic livers, compared to those with cirrhotic livers[319].
The major risk factor for the development of HCC is cirrhosis of the liver. However, about one quarter of HCC cases diagnosed in the United States do not have any known predisposing risk factors. The major known risk factors for HCC are viral (chronic hepatitis B and hepatitis C), toxic (alcohol and aflatoxins), metabolic (diabetes and non-alcoholic fatty liver disease, hereditary haemochromatosis) and immune-related (primary biliary cirrhosis and autoimmune hepatitis)[17]. Recently, the geographical variability in the incidence of HCC has been attributed to the changing distribution and the natural history of Hepatitis B virus (HBV) and Hepatitis C virus (HCV) infection[20].
HCV is the most important risk factor for HCC in western European and North American countries, since epidemiological studies have shown up to 70% of patients with HCC have anti-HCV antibody in the serum[321–23]. Liver cancer has a higher prevalence in patients with HCV-associated cirrhosis than in non-viral aetiologies of chronic liver disease, while only a few cases of HCV-associated HCC have been reported in the non-cirrhotic liver, indicating that the virus possibly has a mutagenic effect[32425].
The prevalence of HCV infection varies considerably by geographical region. African and Asian countries reported high HCV infection prevalence rates, while rates in North America, Europe and Australia have usually reported lower rates[2627]. Egypt has the highest prevalence of HCV in the world[28–34] (predominantly genotype 4), which has been attributed to previous public health eradication schemes for schistosomiasis[2834]. Even higher HCV infection rates, up to 60%, have been reported in older individuals, in rural areas such as the Nile delta, and in lower social classes[28303234].
The natural history of HCV infection has been investigated in several studies[3]. A Japanese study[35] reported a time lag of 13 years from infection by transfusion of HCV infected blood to the development of chronic hepatitis. This time period was reported to be approximately 10 years in an American study and it took about 20 years for the same patients to develop cirrhosis of the liver[36]. Development of HCC took 28 years in the American subjects and 29 years in the Japanese cohort[3536]. The annual risk of developing HCC in HCV-infected patients depends on the presence and severity of the underlying liver disease[3].
Up to 80% of HCV-infected individuals fail to eliminate the virus acutely and progress to chronic HCV infection[2737–40]. Continuous inflammation and hepatocyte regeneration in the setting of chronic hepatitis and subsequent progression to cirrhosis is thought to lead to chromosomal damage and possibly to initiate hepatic carcinogenesis[2741].
The rate of fibrotic progression following HCV infection is markedly variable, since the natural history of the disease typically extends over several decades[4041]. The rate of fibrotic progression in HCV-infected patients is influenced by age at the time of infection, male sex, HCV genotype and alcohol consumption[42–50].
It is not clear whether any of these factors affect the onset of liver-related complications by mechanisms other than their effects on the rate of fibrotic progression. To determine which interactive variables were independent determinants of adverse clinical outcomes, Khan and colleagues examined the development of liver-related complications of chronic HCV in a large cohort of patients who were heterogeneous in age, country of birth, mode of HCV acquisition, HCV genotype, and histological and functional severity of liver disease. Patients were followed up for five years. These authors found that the major independent predictors of liver-related complications were sporadic transmission, advanced liver fibrosis at entry and low albumin[43].
The WHO has reported HBV to be second only to tobacco as a known human carcinogen[51]. Many studies on HCC risk following chronic HBV infection have been conducted in the East Asian countries, where most patients acquired HBV as newborn infants[5253]. The incidence of HCC in HBV-related cirrhosis in this area of the world has been reported to be 2.7%[53]. The annual risk of HCC is 0.5% for asymptomatic HBsAg carriers and 0.8% for patients with chronic hepatitis B[5354], while patients with HBV-cirrhosis have 1000 times higher risk of developing HCC, compared to HBsAg negative individuals[5355]. Thus, it is likely that the probability of acquiring HCC increases with severity of underlying liver disease[53]. In Japan, the mean interval between HBV initial infection and the occurrence of HCC is 50 years. As most people are infected at birth, HBV-related cirrhosis usually develops earlier than in Western Europe or North America[3553].
Few adequate studies have been performed in Europe or North America to address the issue of the incidence of HCC in individuals who are positive for HBsAg. Most of the studies in Western countries are based on small numbers of HBsAg positive patients and/or have not specifically analysed the group of HBsAg carriers. Additionally there is lack of uniformity in the timing of initiation of follow-up monitoring. In a cohort of 350 Western European patients with compensated cirrhosis, followed for about 6 years, the 5-year cumulative incidence of HCC was 6%[535657]. A retrospective analysis of European patients with HBV-related cirrhosis found the 5-year incidence of HCC was 9%, irrespective of HBeAg or HBV DNA status at the time of diagnosis of cirrhosis[5358].
HCC has been the first human cancer amenable to prevention using mass vaccination programmes. From a global perspective, the burden of chronic HBV infection is expected to decline because of the increasing utilisation of HBV immunization, since the early 1980s[205960]. The Taiwanese mass vaccination program against HBV has considerably reduced the rate of HBsAg carrier in children and adolescents and consequently the incidence of childhood HCC[206162]. The average annual incidence of HCC in children aged 6-14 years declined gradually (0.70 per 100 000 children in 1981-1986, 0.57 in 1986-1990 and 0.36 in 1990-1994). A significant decrease in HCC incidence in adults was also observed, 3-4 decades later[2063].
The mechanisms of carcinogenesis in HBV infection have been extensively studied, and a major factor is chronic necroinflammation with subsequent fibrosis and hepatocyte proliferation. However, HCC may occur in HBsAg carriers without cirrhosis. Both HBV and host hepatocytes may contribute to the final pathogenic outcomes, either individually or synergistically. Therefore, it is reasonable to consider that apart from host factors, viral factors are likely involved in HBV-related hepatocarcinogenesis[20].
HBV may encode oncogenic viral proteins that may contribute to hepatocarcinogenesis[20]. For example, HBx is a well-known viral non-structural gene that has roles as a multifunctional regulator modulating gene transcription, as well as controlling cell responses to genotoxic stress, protein degradation, apoptosis, and several signalling pathways[2064–67]. Although the specific mechanisms are still unknown, its critical role in liver malignant transformation has been demonstrated in studies of transgenic mice with HBx overexpression[2068]. HBx protein has been shown to complex the tumor suppressor p53 protein and to suppress its function[536970].
Several viral factors other than viral proteins as viral genotype, BCP mutations in the viral genome and viral load have been associated with hepatocarcinogenesis[20]. Eight HBV genotypes (A-H) have been described, based on genomic sequence divergence[207172] These have distinct geographical and ethnic distributions: genotypes A and D prevail in Africa, Europe, and India; genotypes B and C in Asia; genotype E only in West Africa; and genotype F in Central and South America[2073]. It is reported that HBV genotype affects clinical outcome and treatment responses. For example, in Asia, genotype C is found to be commonly associated with more severe liver disease, cirrhosis and the development of HCC, compared to genotype B[20656874–78] whereas in Western Europe and North America, genotype D is more associated with severe liver disease and a higher incidence of HCC, than genotype A[2079]. In addition to viral genotype, specific viral genomic mutations, particularly the BCP T1762/A1764 mutation, also correlate with HCC risk[208081].
A prospective cohort study with 11 years of follow-up assessed the relationship between HBV viral load and mortality. Viral load was found to be associated with increased mortality from HCC and chronic liver disease in HBV-infected subjects. The relative risk (RR) for HCC mortality in patients with viral load < 105 copies/mL was 1.7 (95% CI, 0.5-5.7), whereas it was 11.2 (95% CI, 3.6-35.0) in patients with viral load > 105 copies/mL[74–76]. Viral load may thus be a useful prognostic tool in HBV infection.
Viral factors in association with the development of HBV-related HCC in young patients seem to be different from their old-aged counterparts[2082]. Tsai and colleagues compared serum viral loads in young (less than 40 years of age) and older (over 40 years) patient groups in 183 HBV-related HCC patients and 202 HBV carriers. These authors found high serum HBV DNA levels were associated with the development of HCC in older patients, rather than those under 40 years[2083]. Another study from Taiwan demonstrated that genotype B was significantly more common in patients with HCC, aged under 50 years, compared to age-matched inactive carriers (80% vs 52%, P = 0.03)[2080]. This predominance was even more striking in younger patients with HCC, with 90% in those under 35 years. Most of these patients did not have cirrhosis. A further Taiwanese study reported that 26 children with HBV-related HCC were documented among 460 HBV carriers during 15 years follow up and genotype B was the major genotype (74%)[2084]. These data suggest that genotype B-HBV may be associated with the development of HCC in young carriers without cirrhosis[20].
Studies of HBV-related HCC in patients without cirrhosis have helped to explain the effect of viral factors in HCC development. Liu et al (2006) examined the role of BCP T1762/A1764 mutation, pre-core A1896 mutation and serum viral load in liver cancer, presenting in the absence of cirrhosis, by comparing 44 patients without cirrhosis, but with HBV-related HCC, to 42 individuals with cirrhosis and HBV-related HCC. These authors found that male gender, BCP T1762/A1764 mutation and viral load greater than 105 copies/mL were independently associated with the risk of HCC development in the absence of cirrhosis. They suggested that viral features predisposing to HCC might be similar between cirrhotic and non-cirrhotic groups[2085].
Recently, pre-S deletion of HBV has been found to be associated with the progression of liver disease and development of HCC in HBV carriers[2086]. PreS deletion mutants hasten the storage of large envelope proteins in hepatocyte cytoplasm which can stimulate cellular promoters by inducing endoplasmic reticulum stress[538788].
The interactions between pre-S deletion, PC mutation and BCP mutation of various stages of chronic HBV infection were investigated in 46 chronic HBV carriers and 106 age-matched carriers with different stages of liver diseases; 38 with chronic hepatitis, 18 with cirrhosis, and 50 individuals with HCC[87]. Logistic regression analysis demonstrated that pre-S deletion and BCP mutation were significantly associated with the development of progressive liver disease. Combinations of mutations, especially the pre-S deletion, rather than single mutation were correlated with a greater risk of progressive liver disease. Sequencing analysis showed that the deleted regions were more common in the 3’ terminus of pre-S1 and the 5’ terminus of pre-S2[2086].
Follow up studies have shown that patients with combined HCV and HBV infection have a higher risk of developing HCC than those with a HCV or HBV alone[35389]. The cumulative risk of developing HCC was 10%, 21%, and 23%, respectively, after 5 years and 16%, 28% and 45%, respectively, after 10 years[390].
The HCC risk in subjects with both infections was investigated in a meta-analysis of 32 epidemiological studies between 1993 and 1997[5391]. The OR for development of HCC in HBsAg positive, anti-HCV/HCV RNA negative subjects was 20.4; in HBsAg negative, anti-HCV/HCV RNA positive subjects, 23.6; and subjects positive for both markers, the OR was 135. These data suggest a more than additive effect of HBV and HCV coinfection on HCC risk. The two viruses may possibly act through common, as well as different, pathways in the carcinogenic process. Given that HBV acts as a cofactor in the development of HCV related cirrhosis and HCC, vaccination of patients with chronic hepatitis C against HBV has been recommended aiming to avoid further liver injury[539293].
HDV coinfection with HBV is associated with increased liver damage. Verme and coworkers showed that HBsAg positive patients with HDV superinfection develop cirrhosis and HCC at an earlier stage (mean age 48 years), compared to HBsAg carriers without HDV infection (mean age 62 years)[5394].
Chronic hepatitis C is more aggressive in HIV positive subjects, leading to cirrhosis and liver failure in a shorter time period[5395]. Coinfection with HIV is a frequent occurrence because of shared routes of transmission. A recent study of HCC in HIV-HCV coinfected patients indicated rapid development of HCC in these patients[5396].
Schistosomiasis is a common parasitic infestation in some parts of the world. In Egypt, Schistosomiasis is a major public health problem and infection with Schistosoma mansoni constitutes the major cause of liver disease. From 1950s until 1980s, the Egyptian Ministry of Health (MOH) conducted a community-wide therapy campaign using parenteral tarter emetic to control the Schistosomiasis infestation. However, this unfortunately established a large reservoir of HCV infection in the country through needle re-usage at the time of treatment[97]. There is some epidemiological evidence that the presence of schistosomal infection may modify the course of hepatitis C genotype 4 co-infection and may lead to significantly more complications, such as portal hypertension at an earlier stage with accelerated progression to hepatitis C-associated fibrosis and thus quicker progression to HCC, than those patients who do not have a parasite burden[1331–34].
AFB1 is produced by a fungus of the genus, Aspergillus spp, in Asia and sub-Saharan Africa in which climatic factors and storage techniques favour the fungus to be a common contaminant of foods, such as grain, corn, peanuts and legumes. Areas with high exposure of AFB1 coincide with areas with a high prevalence of HCC. It has also been suggested that a high intake of AFB1 in HBV-infected patients is an added risk factor for HCC development[3739899]. It has been observed that areas with a high prevalence of HCC and high aflatoxin intake also correspond to areas with endemic HBV infection, and that patients at highest risk of developing HCC are those who are exposed to both HBV and AFB1[398].
Somatic mutations of the tumor suppressor p53 gene are the commonest genetic abnormality in human cancer and evidence supports a high level of p53 alterations in HCC. El Far and colleagues investigated p53 mutations in Egyptian patients with HCC and its relation to other prognostic factors, such as tumor grade, α-fetoprotein (AFP) and liver function tests to elucidate their implication in HCC pathogenesis. These authors found that p53 detection increased the frequency of HCC prediction from 79.5% to 86.3%. Moreover, significant positive correlation between p53 mutation and tumor size for tumor grade II and III was identified. Thus, serum concentration of p53 protein may be a potential non-invasive screening test for predicting risk of HCC[100].
It has been suggested that AFB1 can lead to HCC through inciting a specific mutation of codon 249 of the p53 tumor suppressor gene[101]. However, this mutation has also been found in patients who had previous contact with the HBV[378].
Pesticides exposure is one of the environmental factors hypothesized to increase the risk of HCC. Pesticides are considered to be possible epigenetic carcinogens through one or several mechanisms, such as spontaneous initiation of genetic changes, cytotoxicity with persistent cell proliferation, oxidative stress, inhibition of apoptosis, suppression of intracellular communication and construction of activated receptors[102103].
A case-control study of HCC in HBV and/or HCV infected patients from Egypt suggested pesticides had an additive effect on the risk of HCC in rural males, amongst whom the use of carbamate and organophosphate compounds is commonplace[103].
A population-based study from the USA found diabetes to be an independent risk factor for HCC, regardless of chronic HCV or HBV infection, alcoholic liver disease, or non-specific cirrhosis. Diabetes was associated with a two- to threefold increase in HCC risk. About 60% of patients with HCC in this study were not diagnosed with chronic HCV-related or HBV-related hepatitis, alcoholic liver disease, or other known causes of chronic liver disease. Among these patients, 47% had diabetes, which was higher than those with other risk factors (41%). This suggests that diabetes may represent a considerable proportion of patients with idiopathic HCC[104].
An increased risk of HCC among patients with diabetes alone was also reported in a population based study using data obtained from the Denmark cancer registry[104105]. Also, it is reported a threefold increased risk of liver cancer among patients hospitalized with diabetes, as well as and a fourfold risk in the presence of hepatitis, cirrhosis, and alcoholism in a Swedish study[104106].
Diabetes, as part of the insulin resistance syndrome, has been implicated as a risk factor for non-alcoholic fatty liver disease (NAFLD), including in its most severe form, non-alcoholic steatohepatitis (NASH). NASH has been identified as a cause of both “cryptogenic cirrhosis” and HCC[104107–112].
Many epidemiological studies have examined the relationship between diet and HCC risk[113]. The results are somewhat conflicting. Some studies have shown an inverse relationship between HCC and diets which are high in milk, wheat, vegetable, fish and fruit content. Other studies have shown no association.
With regard to egg consumption, two studies reported an inverse relation with HCC risk[114115], while three others reported an increased risk[115–117]. Similarly, two studies[118119] demonstrated that meat and animal protein consumption were associated with increased risk of HCC, although other studies[114–116] did not support this finding[113].
To verify if consumption of soya foods reduce the HCC risk, Sharp and his colleagues conducted a case-control study within a cohort of Japanese A-bomb survivors. They compared the pre-diagnosis intake of isoflavone-rich miso soup and tofu to HCC risk, adjusting for hepatitis B (HBV) and C (HCV) viral infections. They concluded that consumption of miso soup and other soya foods may reduce HCC risk and this is consistent with the results of epidemiological, animal and laboratory-based studies, as well as some clinical trials[120]. This is explained by the opposing effect of isoflavones on oestrogen and testosterone levels which reduce HCC risk, possibly by modifying the hormonal profile and reducing cell proliferation, associated with increased cancer risk. An alternative explanation may be that isoflavones provide an independent anti-tumour effect, such as suppression of angiogenesis, or stimulation of apoptosis[120].
A 41% reduction in HCC risk among coffee drinkers, compared to non-drinkers, has been observed in a meta-analysis study[121]. This favourable effect of coffee drinking was established both in studies from southern Europe[122–124], where coffee is widely consumed, and from Japan[125126], where coffee intake is less frequent, and in subjects with chronic liver disease[121]. Some compounds in coffee, including diterpenes, cafestol, and kahweol, may act as blocking agents via modulation of multiple enzymes involved in carcinogenic detoxification as demonstrated in animal models and cell culture systems[119127128]. Moreover, coffee components modify the xenotoxic metabolism via induction of glutathione-S-transferase and inhibition of N-acetyltransferase[121129]. Other components of coffee, including caffeine and antioxidant substances from coffee beans, have been related to favorable modifications in liver enzymes such as γ-glutamyltransferase and aminotransferase activities[119130–133].
HCC is one of the commonest cancers worldwide. It is a major health problem and its incidence is increasing. The presence of cirrhosis is the major risk factor and worldwide this is largely due to chronic HCV and HBV infection. HCC carcinogenesis is likely to involve interplay of viral, environmental and host factors. The advent of mass-vaccination programmes for hepatitis B, particularly in East Asia is beginning to reduce prevalence rates for HCC in some countries, but for the most part, HCV-related HCC is increasing. Concerted strategies need to be developed for HCC surveillance in at risk populations.
| 1. | World Health Organization. Mortality database. Available from: URL: http://www.who.int/whosis/en. [Cited in This Article: ] |
| 2. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827-841. [Cited in This Article: ] |
| 3. | Montalto G, Cervello M, Giannitrapani L, Dantona F, Terranova A, Castagnetta LA. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:13-20. [Cited in This Article: ] |
| 4. | Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143-154. [Cited in This Article: ] |
| 5. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [Cited in This Article: ] |
| 6. | Yeh CT, Chen TC, Chang ML, Hsu CW, Yeh TS, Lee WC, Huang SF, Tsai CC. Identification of NV-F virus DNA in hepatocellular carcinoma. J Med Virol. 2007;79:92-96. [Cited in This Article: ] |
| 7. | Ferlay J, Parkin DM, Pisani P. Globocan Graphical Package 1: Cancer Incidence and Mortality Worldwide. Lyon: IARC Press 1998; . [Cited in This Article: ] |
| 8. | Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [Cited in This Article: ] |
| 9. | Di Bisceglie AM. Epidemiology and clinical presentation of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S169-S171. [Cited in This Article: ] |
| 10. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [Cited in This Article: ] |
| 11. | Taylor-Robinson SD, Thomas HC, Arora S, Hargreaves S. Increased mortality from liver cancer in England and Wales is not related to hepatitis C. BMJ. 1999;319:640. [Cited in This Article: ] |
| 12. | Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813. [Cited in This Article: ] |
| 13. | el-Zayadi AR, Badran HM, Barakat EM, Attia Mel-D, Shawky S, Mohamed MK, Selim O, Saeid A. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol. 2005;11:5193-5198. [Cited in This Article: ] |
| 14. | Jong-wook L. Global health improvement and WHO: shaping the future. Lancet. 2003;362:2083-2088. [Cited in This Article: ] |
| 15. | Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene. 2006;25:3771-3777. [Cited in This Article: ] |
| 16. | El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37 Suppl 2:S88-S94. [Cited in This Article: ] |
| 17. | Parikh S, Hyman D. Hepatocellular cancer: a guide for the internist. Am J Med. 2007;120:194-202. [Cited in This Article: ] |
| 18. | Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [Cited in This Article: ] |
| 20. | Liu CJ, Kao JH. Hepatitis B virus-related hepatocellular carcinoma: epidemiology and pathogenic role of viral factors. J Chin Med Assoc. 2007;70:141-145. [Cited in This Article: ] |
| 21. | Nishioka K, Watanabe J, Furuta S, Tanaka E, Iino S, Suzuki H, Tsuji T, Yano M, Kuo G, Choo QL. A high prevalence of antibody to the hepatitis C virus in patients with hepatocellular carcinoma in Japan. Cancer. 1991;67:429-433. [Cited in This Article: ] |
| 22. | Bruix J, Barrera JM, Calvet X, Ercilla G, Costa J, Sanchez-Tapias JM, Ventura M, Vall M, Bruguera M, Bru C. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004-1006. [Cited in This Article: ] |
| 23. | Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006-1008. [Cited in This Article: ] |
| 24. | De Mitri MS, Poussin K, Baccarini P, Pontisso P, D'Errico A, Simon N, Grigioni W, Alberti A, Beaugrand M, Pisi E. HCV-associated liver cancer without cirrhosis. Lancet. 1995;345:413-415. [Cited in This Article: ] |
| 25. | el-Refaie A, Savage K, Bhattacharya S, Khakoo S, Harrison TJ, el-Batanony M, Soliman el-S, Nasr S, Mokhtar N, Amer K. HCV-associated hepatocellular carcinoma without cirrhosis. J Hepatol. 1996;24:277-285. [Cited in This Article: ] |
| 26. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [Cited in This Article: ] |
| 27. | Suruki RY, Mueller N, Hayashi K, Harn D, DeGruttola V, Raker CA, Tsubouchi H, Stuver SO. Host immune status and incidence of hepatocellular carcinoma among subjects infected with hepatitis C virus: a nested case-control study in Japan. Cancer Epidemiol Biomarkers Prev. 2006;15:2521-2525. [Cited in This Article: ] |
| 28. | Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887-891. [Cited in This Article: ] |
| 29. | Hibbs RG, Corwin AL, Hassan NF, Kamel M, Darwish M, Edelman R, Constantine NT, Rao MR, Khalifa AS, Mokhtar S. The epidemiology of antibody to hepatitis C in Egypt. J Infect Dis. 1993;168:789-790. [Cited in This Article: ] |
| 30. | el Gohary A, Hassan A, Nooman Z, Lavanchy D, Mayerat C, el Ayat A, Fawaz N, Gobran F, Ahmed M, Kawano F. High prevalence of hepatitis C virus among urban and rural population groups in Egypt. Acta Trop. 1995;59:155-161. [Cited in This Article: ] |
| 31. | Darwish NM, Abbas MO, Abdelfattah FM, Darwish MA. Hepatitis C virus infection in blood donors in Egypt. J Egypt Public Health Assoc. 1992;67:223-236. [Cited in This Article: ] |
| 32. | Darwish MA, Raouf TA, Rushdy P, Constantine NT, Rao MR, Edelman R. Risk factors associated with a high seroprevalence of hepatitis C virus infection in Egyptian blood donors. Am J Trop Med Hyg. 1993;49:440-447. [Cited in This Article: ] |
| 33. | Kamel MA, Ghaffar YA, Wasef MA, Wright M, Clark LC, Miller FD. High HCV prevalence in Egyptian blood donors. Lancet. 1992;340:427. [Cited in This Article: ] |
| 34. | Hassan MM, Zaghloul AS, El-Serag HB, Soliman O, Patt YZ, Chappell CL, Beasley RP, Hwang LY. The role of hepatitis C in hepatocellular carcinoma: a case control study among Egyptian patients. J Clin Gastroenterol. 2001;33:123-126. [Cited in This Article: ] |
| 35. | Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell RH. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671-675. [Cited in This Article: ] |
| 36. | Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463-1466. [Cited in This Article: ] |
| 37. | Tsai SL, Liaw YF, Chen MH, Huang CY, Kuo GC. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology. 1997;25:449-458. [Cited in This Article: ] |
| 38. | Sarih M, Bouchrit N, Benslimane A. Different cytokine profiles of peripheral blood mononuclear cells from patients with persistent and self-limited hepatitis C virus infection. Immunol Lett. 2000;74:117-120. [Cited in This Article: ] |
| 39. | Cucchiarini M, Kammer AR, Grabscheid B, Diepolder HM, Gerlach TJ, Gruner N, Santantonio T, Reichen J, Pape GR, Cerny A. Vigorous peripheral blood cytotoxic T cell response during the acute phase of hepatitis C virus infection. Cell Immunol. 2000;203:111-123. [Cited in This Article: ] |
| 40. | Gruner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, Hoffmann R, Zachoval R, Santantonio T, Cucchiarini M. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528-1536. [Cited in This Article: ] |
| 42. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [Cited in This Article: ] |
| 43. | Khan MH, Farrell GC, Byth K, Lin R, Weltman M, George J, Samarasinghe D, Kench J, Kaba S, Crewe E. Which patients with hepatitis C develop liver complications? Hepatology. 2000;31:513-520. [Cited in This Article: ] |
| 44. | Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, Hollinger FB, Gitnick G, Knodell RG, Perrillo RP. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992;327:1906-1911. [Cited in This Article: ] |
| 45. | Delladetsima JK, Rassidakis G, Tassopoulos NC, Papatheodoridis GV, Smyrnoff T, Vafiadis I. Histopathology of chronic hepatitis C in relation to epidemiological factors. J Hepatol. 1996;24:27-32. [Cited in This Article: ] |
| 46. | Kobayashi M, Tanaka E, Sodeyama T, Urushihara A, Matsumoto A, Kiyosawa K. The natural course of chronic hepatitis C: a comparison between patients with genotypes 1 and 2 hepatitis C viruses. Hepatology. 1996;23:695-699. [Cited in This Article: ] |
| 47. | Shev S, Dhillon AP, Lindh M, Serleus Z, Wejstal R, Widell A, Norkrans G. The importance of cofactors in the histologic progression of minimal and mild chronic hepatitis C. Liver. 1997;17:215-223. [Cited in This Article: ] |
| 48. | Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology. 1998;27:1730-1735. [Cited in This Article: ] |
| 49. | Khan MH, Thomas L, Byth K, Kench J, Weltman M, George J, Liddle C, Farrell GC. How much does alcohol contribute to the variability of hepatic fibrosis in chronic hepatitis C? J Gastroenterol Hepatol. 1998;13:419-426. [Cited in This Article: ] |
| 50. | Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-809. [Cited in This Article: ] |
| 51. | WHO. Department of Communable Diseases, Surveillance and Response: WHO. Hepatitis B. 2002;. [Cited in This Article: ] |
| 52. | Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976;294:746-749. [Cited in This Article: ] |
| 53. | Michielsen PP, Francque SM, van Dongen JL. Viral hepatitis and hepatocellular carcinoma. World J Surg Oncol. 2005;3:27. [Cited in This Article: ] |
| 54. | Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, Pao CC. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology. 1986;90:263-267. [Cited in This Article: ] |
| 55. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [Cited in This Article: ] |
| 56. | Realdi G, Fattovich G, Hadziyannis S, Schalm SW, Almasio P, Sanchez-Tapias J, Christensen E, Giustina G, Noventa F. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21:656-666. [Cited in This Article: ] |
| 57. | Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, Christensen E, Krogsgaard K, Degos F, Carneiro de Moura M. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21:77-82. [Cited in This Article: ] |
| 58. | Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97:2886-2895. [Cited in This Article: ] |
| 59. | Margolis HS. Hepatitis B virus infection. Bull World Health Organ. 1998;76 Suppl 2:152-153. [Cited in This Article: ] |
| 60. | Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13 Suppl 1:S47-S49. [Cited in This Article: ] |
| 61. | Ni YH, Chang MH, Huang LM, Chen HL, Hsu HY, Chiu TY, Tsai KS, Chen DS. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135:796-800. [Cited in This Article: ] |
| 62. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [Cited in This Article: ] |
| 63. | Kao JH, Chen DS. Changing disease burden of hepatocellular carcinoma in the Far East and Southeast Asia. Liver Int. 2005;25:696-703. [Cited in This Article: ] |
| 64. | Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651-660. [Cited in This Article: ] |
| 65. | Arbuthnot P, Capovilla A, Kew M. Putative role of hepatitis B virus X protein in hepatocarcinogenesis: effects on apoptosis, DNA repair, mitogen-activated protein kinase and JAK/STAT pathways. J Gastroenterol Hepatol. 2000;15:357-368. [Cited in This Article: ] |
| 66. | Oda T, Tsuda H, Scarpa A, Sakamoto M, Hirohashi S. p53 gene mutation spectrum in hepatocellular carcinoma. Cancer Res. 1992;52:6358-6364. [Cited in This Article: ] |
| 67. | de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847-8851. [Cited in This Article: ] |
| 68. | Wu BK, Li CC, Chen HJ, Chang JL, Jeng KS, Chou CK, Hsu MT, Tsai TF. Blocking of G1/S transition and cell death in the regenerating liver of Hepatitis B virus X protein transgenic mice. Biochem Biophys Res Commun. 2006;340:916-928. [Cited in This Article: ] |
| 69. | Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci USA. 1994;91:2230-2234. [Cited in This Article: ] |
| 70. | Truant R, Antunovic J, Greenblatt J, Prives C, Cromlish JA. Direct interaction of the hepatitis B virus HBx protein with p53 leads to inhibition by HBx of p53 response element-directed transactivation. J Virol. 1995;69:1851-1859. [Cited in This Article: ] |
| 71. | Norder H, Courouce AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994;198:489-503. [Cited in This Article: ] |
| 72. | Okamoto H, Kurai K, Okada S, Yamamoto K, Lizuka H, Tanaka T, Fukuda S, Tsuda F, Mishiro S. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992;188:331-341. [Cited in This Article: ] |
| 73. | Liu CJ, Kao JH, Chen DS. Therapeutic implications of hepatitis B virus genotypes. Liver Int. 2005;25:1097-1107. [Cited in This Article: ] |
| 74. | Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol. 2006;101:1797-1803. [Cited in This Article: ] |
| 75. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [Cited in This Article: ] |
| 76. | Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11:797-816, viii. [Cited in This Article: ] |
| 77. | Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023-2032. [Cited in This Article: ] |
| 78. | Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, Liu CJ, Shih WL, Kao JH, Chen DS, Chen CJ. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97:265-272. [Cited in This Article: ] |
| 79. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [Cited in This Article: ] |
| 80. | Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology. 2003;124:327-334. [Cited in This Article: ] |
| 81. | Minami M, Poussin K, Kew M, Okanoue T, Brechot C, Paterlini P. Precore/core mutations of hepatitis B virus in hepatocellular carcinomas developed on noncirrhotic livers. Gastroenterology. 1996;111:691-700. [Cited in This Article: ] |
| 82. | Ou LH, Chau GY, Tsay SH, Chiu JH, Wu JC, King KL, Loong CC, Wu CW, Lui WY. Clinicopathological comparison of resectable hepatocellular carcinoma between the young and the elderly patients. Zhonghua Yi Xue Za Zhi (Taipei). 1997;60:40-47. [Cited in This Article: ] |
| 83. | Tsai FC, Liu CJ, Chen CL, Chen PJ, Lai MY, Kao JH, Chen DS. Lower serum viral loads in young patients with hepatitis-B-virus-related hepatocellular carcinoma. J Viral Hepat. 2007;14:153-160. [Cited in This Article: ] |
| 84. | Ni YH, Chang MH, Wang KJ, Hsu HY, Chen HL, Kao JH, Yeh SH, Jeng YM, Tsai KS, Chen DS. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004;127:1733-1738. [Cited in This Article: ] |
| 85. | Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, Kao JH, Chen DS. Role of hepatitis B virus precore/core promoter mutations and serum viral load on noncirrhotic hepatocellular carcinoma: a case-control study. J Infect Dis. 2006;194:594-599. [Cited in This Article: ] |
| 86. | Chen BF, Liu CJ, Jow GM, Chen PJ, Kao JH, Chen DS. High prevalence and mapping of pre-S deletion in hepatitis B virus carriers with progressive liver diseases. Gastroenterology. 2006;130:1153-1168. [Cited in This Article: ] |
| 87. | Xu Z, Jensen G, Yen TS. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J Virol. 1997;71:7387-7392. [Cited in This Article: ] |
| 88. | Bock CT, Tillmann HL, Manns MP, Trautwein C. The pre-S region determines the intracellular localization and appearance of hepatitis B virus. Hepatology. 1999;30:517-525. [Cited in This Article: ] |
| 89. | Sato S, Fujiyama S, Tanaka M, Yamasaki K, Kuramoto I, Kawano S, Sato T, Mizuno K, Nonaka S. Coinfection of hepatitis C virus in patients with chronic hepatitis B infection. J Hepatol. 1994;21:159-166. [Cited in This Article: ] |
| 90. | Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132-2137. [Cited in This Article: ] |
| 91. | Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347-354. [Cited in This Article: ] |
| 92. | National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology. 1997;26:2S-10S. [Cited in This Article: ] |
| 93. | Chlabicz S, Grzeszczuk A. Hepatitis B virus vaccine for patients with hepatitis C virus infection. Infection. 2000;28:341-345. [Cited in This Article: ] |
| 94. | Verme G, Brunetto MR, Oliveri F, Baldi M, Forzani B, Piantino P, Ponzetto A, Bonino F. Role of hepatitis delta virus infection in hepatocellular carcinoma. Dig Dis Sci. 1991;36:1134-1136. [Cited in This Article: ] |
| 95. | Telfer P, Sabin C, Devereux H, Scott F, Dusheiko G, Lee C. The progression of HCV-associated liver disease in a cohort of haemophilic patients. Br J Haematol. 1994;87:555-561. [Cited in This Article: ] |
| 96. | Garcia-Samaniego J, Rodriguez M, Berenguer J, Rodriguez-Rosado R, Carbo J, Asensi V, Soriano V. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:179-183. [Cited in This Article: ] |
| 97. | Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43:915-922. [Cited in This Article: ] |
| 98. | Groopman JD, Scholl P, Wang JS. Epidemiology of human aflatoxin exposures and their relationship to liver cancer. Prog Clin Biol Res. 1996;395:211-222. [Cited in This Article: ] |
| 99. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [Cited in This Article: ] |
| 100. | El Far MA, Atwa MA, Yahya RS, El Basuni MA. Evaluation of serum levels of p53 in hepatocellular carcinoma in Egypt. Clin Chem Lab Med. 2006;44:653-656. [Cited in This Article: ] |
| 101. | Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429-431. [Cited in This Article: ] |
| 102. | Rakitsky VN, Koblyakov VA, Turusov VS. Nongenotoxic (epigenetic) carcinogens: pesticides as an example. A critical review. Teratog Carcinog Mutagen. 2000;20:229-240. [Cited in This Article: ] |
| 103. | Ezzat S, Abdel-Hamid M, Eissa SA, Mokhtar N, Labib NA, El-Ghorory L, Mikhail NN, Abdel-Hamid A, Hifnawy T, Strickland GT. Associations of pesticides, HCV, HBV, and hepatocellular carcinoma in Egypt. Int J Hyg Environ Health. 2005;208:329-339. [Cited in This Article: ] |
| 104. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [Cited in This Article: ] |
| 105. | Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360-1365. [Cited in This Article: ] |
| 106. | Adami HO, Chow WH, Nyren O, Berne C, Linet MS, Ekbom A, Wolk A, McLaughlin JK, Fraumeni JF Jr. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472-1477. [Cited in This Article: ] |
| 107. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. [Cited in This Article: ] |
| 108. | Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20:594-598. [Cited in This Article: ] |
| 109. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [Cited in This Article: ] |
| 110. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [Cited in This Article: ] |
| 111. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [Cited in This Article: ] |
| 112. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [Cited in This Article: ] |
| 113. | Talamini R, Polesel J, Montella M, Dal Maso L, Crispo A, Tommasi LG, Izzo F, Crovatto M, La Vecchia C, Franceschi S. Food groups and risk of hepatocellular carcinoma: A multicenter case-control study in Italy. Int J Cancer. 2006;119:2916-2921. [Cited in This Article: ] |
| 114. | La Vecchia C, Negri E, Decarli A, D’Avanzo B, Franceschi S. Risk factors for hepatocellular carcinoma in northern Italy. Int J Cancer. 1988;42:872-876. [Cited in This Article: ] |
| 115. | Yu SZ, Huang XE, Koide T, Cheng G, Chen GC, Harada K, Ueno Y, Sueoka E, Oda H, Tashiro F. Hepatitis B and C viruses infection, lifestyle and genetic polymorphisms as risk factors for hepatocellular carcinoma in Haimen, China. Jpn J Cancer Res. 2002;93:1287-1292. [Cited in This Article: ] |
| 116. | Fukuda K, Shibata A, Hirohata I, Tanikawa K, Yamaguchi G, Ishii M. A hospital-based case-control study on hepatocellular carcinoma in Fukuoka and Saga Prefectures, northern Kyushu, Japan. Jpn J Cancer Res. 1993;84:708-714. [Cited in This Article: ] |
| 117. | Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, Sakata R, Fujita Y, Ichikawa S, Iwai N. Dietary habits and risk of death due to hepatocellular carcinoma in a large scale cohort study in Japan. Univariate analysis of JACC study data. Kurume Med J. 2004;51:141-149. [Cited in This Article: ] |
| 118. | Braga C, La Vecchia C, Negri E, Franceschi S. Attributable risks for hepatocellular carcinoma in northern Italy. Eur J Cancer. 1997;33:629-634. [Cited in This Article: ] |
| 119. | Yu MW, Chen CJ. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res. 1993;53:790-794. [Cited in This Article: ] |
| 120. | Sharp GB, Lagarde F, Mizuno T, Sauvaget C, Fukuhara T, Allen N, Suzuki G, Tokuoka S. Relationship of hepatocellular carcinoma to soya food consumption: a cohort-based, case-control study in Japan. Int J Cancer. 2005;115:290-295. [Cited in This Article: ] |
| 121. | Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, Franceschi S, La Vecchia C. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430-435. [Cited in This Article: ] |
| 122. | Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498-502. [Cited in This Article: ] |
| 123. | La Vecchia C, Ferraroni M, Negri E, D’Avanzo B, Decarli A, Levi F, Franceschi S. Coffee consumption and digestive tract cancers. Cancer Res. 1989;49:1049-1051. [Cited in This Article: ] |
| 124. | Montella M, Polesel J, La Vecchia C, Dal Maso L, Crispo A, Crovatto M, Casarin P, Izzo F, Tommasi LG, Talamini R. Coffee and tea consumption and risk of hepatocellular carcinoma in Italy. Int J Cancer. 2007;120:1555-1559. [Cited in This Article: ] |
| 125. | Ohfuji S, Fukushima W, Tanaka T, Habu D, Tamori A, Sakaguchi H, Takeda T, Kawada N, Seki S, Nishiguchi S. Coffee consumption and reduced risk of hepatocellular carcinoma among patients with chronic type C liver disease: A case-control study. Hepatol Res. 2006;36:201-208. [Cited in This Article: ] |
| 126. | Tanaka K, Hara M, Sakamoto T, Higaki Y, Mizuta T, Eguchi Y, Yasutake T, Ozaki I, Yamamoto K, Onohara S. Inverse association between coffee drinking and the risk of hepatocellular carcinoma: a case-control study in Japan. Cancer Sci. 2007;98:214-218. [Cited in This Article: ] |
| 127. | Cavin C, Holzhauser D, Constable A, Huggett AC, Schilter B. The coffee-specific diterpenes cafestol and kahweol protect against aflatoxin B1-induced genotoxicity through a dual mechanism. Carcinogenesis. 1998;19:1369-1375. [Cited in This Article: ] |
| 128. | Majer BJ, Hofer E, Cavin C, Lhoste E, Uhl M, Glatt HR, Meinl W, Knasmuller S. Coffee diterpenes prevent the genotoxic effects of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and N-nitrosodimethylamine in a human derived liver cell line (HepG2). Food Chem Toxicol. 2005;43:433-441. [Cited in This Article: ] |
| 129. | Huber WW, Parzefall W. Modification of N-acetyltransferases and glutathione S-transferases by coffee components: possible relevance for cancer risk. Methods Enzymol. 2005;401:307-341. [Cited in This Article: ] |
| 130. | La Vecchia C. Coffee, liver enzymes, cirrhosis and liver cancer. J Hepatol. 2005;42:444-446. [Cited in This Article: ] |
| 131. | Honjo S, Kono S, Coleman MP, Shinchi K, Sakurai Y, Todoroki I, Umeda T, Wakabayashi K, Imanishi K, Nishikawa H. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J Clin Epidemiol. 2001;54:823-829. [Cited in This Article: ] |
| 132. | Nakanishi N, Nakamura K, Nakajima K, Suzuki K, Tatara K. Coffee consumption and decreased serum gamma-glutamyltransferase: a study of middle-aged Japanese men. Eur J Epidemiol. 2000;16:419-423. [Cited in This Article: ] |
| 133. | Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24-32. [Cited in This Article: ] |
| 134. | Ferlay J, Bray F, Pisani P and Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5. version 2.0, IARCPress, Lyon, 2004. Available from: URL: http://www-dep.iarc.fr/. [Cited in This Article: ] |