Published online Dec 14, 2008. doi: 10.3748/wjg.14.7127
Revised: September 27, 2008
Accepted: October 4, 2008
Published online: December 14, 2008
AIM: To investigate whether bone marrow-derived dendritic cells pulsed with tumor lysates induce immunity against gastric cancer ex vivo.
METHODS: c-kit+ hematopoietic progenitor cells were magnetically isolated with a MiniMACS separator from BALB/c mice bone marrow cells. These cells were cultured with cytokines GM-CSF, IL-4, and TNFα to induce their maturation. They were analysed by morphological observation, phenotype analysis, and mixed lymphocyte reaction (MLR). Bone marrow-derived DCs (BM-DCs) were pulsed with tumor cell lysate obtained by rapid freezing and thawing at a 1:3 DC:tumor cell ratio. Finally, cytotoxic T lymphocyte (CTL) activity and interferon gamma (IFNγ) secretion was evaluated ex vivo.
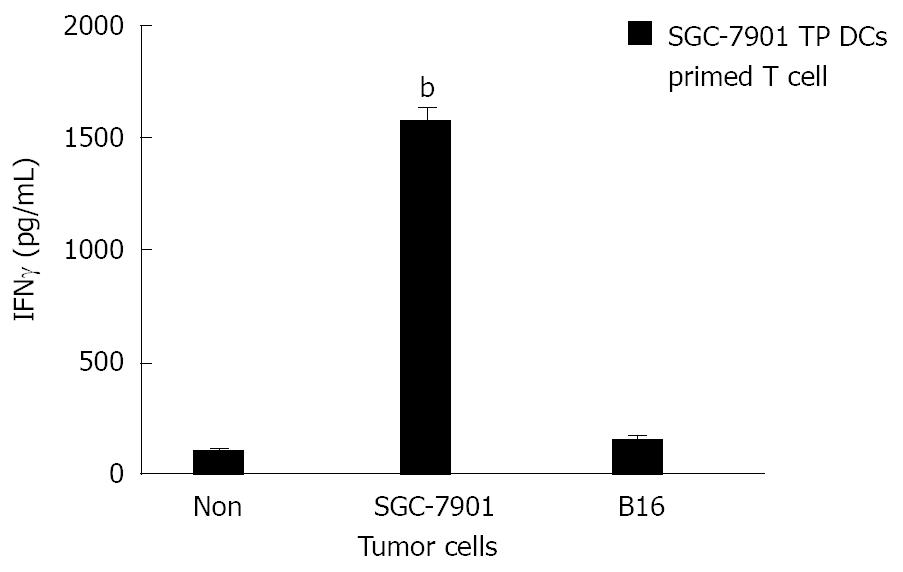
RESULTS: c-kit+ hematopoietic progenitor cells from mice bone marrow cells cultured with cytokines for 8 d showed the character of typical mature DCs. Morphologically, observed by light microscope, these cells were large with oval or irregularly shaped nuclei and with many small dendrites. Phenotypically, FACS analysis showed that they expressed high levels of Ia, DEC-205, CD11b, CD80 and CD86 antigen, moderate levels of CD40, and negative for F4/80. Functionally, these cells gained the capacity to stimulate allogeneic T cells in MLR assay. However, immature DCs cultured with cytokines for 5 d did not have typical DCs phenotypic markers and could not stimulate allogeneic T cells. Ex vivo primed T cells with SGC-7901 tumor cell lysate-pulsed (TP) DCs were able to induce effective CTL activity against SGC-7901 tumor cells (E:T = 100:1, 69.55% ± 6.05% specific lysis), but not B16 tumor cells, and produced higher levels of IFNγ when stimulated with SGC-7901 tumor cells but not when stimulated with B16 tumor cells (1575.31 ± 60.25 pg/mL in SGC-7901 group vs 164.11 ± 18.52 pg/mL in B16 group, P < 0.01).
CONCLUSION: BM-derived DCs pulsed with tumor lysates can induce anti-tumor immunity specific to gastric cancer ex vivo.
-
Citation: Li YL, Wu YG, Wang YQ, Li Z, Wang RC, Wang L, Zhang YY. Bone marrow-derived dendritic cells pulsed with tumor lysates induce anti-tumor immunity against gastric cancer
ex vivo . World J Gastroenterol 2008; 14(46): 7127-7132 - URL: https://www.wjgnet.com/1007-9327/full/v14/i46/7127.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7127
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that both initiate and modulate the immune response[1]. DCs are cells in the pathway of antigen capture and presentation to T cells, with the unique ability to directly prime naïve CD4+ and CD8+ T cells. They posses the ability to efficiently uptake, process, and present antigens on major histocompatibility complex (MHC) class I and II molecules, together with co-stimulatory molecules such as B7 and CD40[2]. Therefore, DCs are believed to be essential for stimulating tumor-specific cytotoxic T lymphocyte (CTL) and inducing the protective and therapeutic anti-tumor immunity against cancer cells.
Gastric cancer is one of the most common cancers[3]. Although gastric cancer therapy has made great progress, it is still difficult to treat advanced gastric cancer, as it has spread to the lymph glands and metastasized. Currently, radical surgery represents the standard method of therapy. Adjuvant therapy such as chemotherapy and radiation therapy have been widely applied, but gastric cancer control at the advanced stage remains difficult[4,5]. A new adjuvant therapeutic method is needed in order to improve the 5-year survival rate of patients with gastric cancer. Currently, tumor immunotherapy for gastric cancer has potential.
Ishigami et al[6] examined 169 patients with gastric cancer by immunohistochemical staining of CD57 and S-100-protein and found that DCs infiltrate in the tissue of gastric caner, but cannot play an important role due to lacking Th cells in the tumor microenvironment. In addition, a poorer differentiation of gastric cancer corresponds to a the lower amount of DC infiltration in the tumor tissue. Patients with a high level of DCs infiltration had a lower positivity of lymph node metastases and lymphatic invasion than patients with lower level of DCs infiltration[7]. The 5-year survival rates of patients with many DCs infiltrated were 78% better than that of patients with fewer DCs infiltrated[6]. According to the function of DCs described above, we can state that the DCs are related to clinical stage, invasion, metastasis and prognosis of gastric cancer. Galetto et al[8] reported that T-cell memory against gastric carcinoma antigens can be triggered by tumor-loaded autologous DCs. Therefore, it is feasible that DCs-based tumor vaccines will become a new effective immunoadjuvant therapy for gastric cancer, which can decrease the incidence and recurrence rate after operation for gastric cancer[9].
In this study, we demonstrated that vaccination with bone marrow-derived DCs pulsed with tumor cell lysate induced tumor-specific CTL activity and anti-tumor immunity against SGC-7901 gastric cancer cell lines, suggesting promising strategy for gastric cancer immunotherapy.
BALB/c and C57BL/6 (B6) mice (8-10 wk old) were purchased from the Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). All mice were kept under pathogen-free conditions in the animal center of the Soochow University (Suzhou, China).
Gastric cancer cell line, SGC-7901, and melanoma cell line, B16, were purchased from the Shanghai Cell Biology Institutes, Chinese Academy of Sciences (Shanghai, China). Both cell lines were cultured in RPMI (Roswell Park Memorial Institute) medium 1640 (GIBCO, USA) containing 10% fetal calf serum (FCS), penicillin G (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified incubator supplemented with 50 mL/L CO2.
Murine granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, tumor necrosis factor-α (TNFα), IL-2, and IL-7 were purchased from Becton Dickinson (New Jersey, USA). Phenotypic analysis, fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled monoclonal antibodies (Mabs), such as MHC I (Ia), DEC-205, CD11b, CD40, CD80, CD86, and F4/80, were provided by Pharmigen (CA, USA). Mitomycin C (MMC) was purchased from Jingmei Biothe (Shenzhen, China).
Primary bone marrow-derived DCs (BM-DCs) were obtained from mouse bone marrow precursors according to a previously established protocol[10]. BALB/c mice bone marrow obtained from tibia and femurs by flushing with media. Tissue was minced in a single-cell suspension through a nylon mesh. BALB/c mice bone marrow cells were stained sequentially with biotinylated anti-c-kit MAb and Streptavidm MicroBeads (Dynal, Norway). c-kit+ hematopoietic progenitor cells were magnetically isolated with a MiniMACS separator (Milteyi Biotec, Auburn, CA) from the BALB/c mice bone marrow cells. The cells (6 × 106) were cultured for 5 d in fresh RPMI medium 1640 containing 10% FCS, GM-CSF (4 ng/mL), and IL-4 (10 ng/mL). Then immature DCs were further cultured in GM-CSF (4 ng/mL) and TNFα (5 ng/mL) for 3 d to induce their maturation. Bone marrow-derived mature DCs were observed by light microscope (Nikon, Japan).
DCs immunofluorescence analysis was performed as previously described[11]. In brief, DCs cultured for 5 d or 8 d as described above (2 × 105-4 × 105 cells) were incubated with rat anti-DEC-205 MAb followed by FITC-labeled goat anti-rat IgG (Fab’)2 antibodies or directly with FITC-labeled MAbs against CD40, F4/80, CD11b, or CD80 and PE-labeled MAbs against Ia, or CD86 followed by FACS analysis. The instrument compensation was set in each experiment using two-color stained samples.
The mixed leukocyte reaction (MLR) assay was performed in accordance with previously described methods[12]. Immature DCs or mature DCs derived from bone marrow c-kit+ cells were incubated in RPMI Medium 1640 containing 10% FCS and mitomycin C (MMC; 15 μg/mL) in six-well plates at 37°C for 3 h to arrest their proliferation. After several washes with PBS, these stimulator cells were suspended in RPMI-1640 containing 10% FCS at concentrations ranging from 1 × 102 to 5 × 104 cells/mL. One hundred microliters of the above stimulator cells suspension were added to each well of 96-well plates that contained allogeneic CD4+ T cells (3 × 105 cells/100 μL per well) that had been magnetically isolated from B6 mice using CD4 Microbeads. Five days later, T-cell proliferation was determined by using an MTT assay. Fifteen μL of MTT (5 μg/mL in PBS) was added to each well, and the plates were incubated at 37°C for an additional 4 h. The resultant absorbance at 550 nm was read by a microplate immunoreader. PBS alone was used as the negative control. Results are expressed as the mean of three wells from three individual experiments.
BM-DCs were pulsed with freeze-thawed tumor lysate at a 1:3 DC:tumor cell ratio. SGC-7901 tumor cells (6 × 106) were lysed by rapid freezing (liquid nitrogen) and thawing in a 37°C bath three times. BM-DCs (2 × 106 cells/mL) were incubated in six-well plates in the presence of SGC7901 tumor lysates (6 × 106 cell equivalents/mL) in RPMI Medium 1640 containing 10% FCS, GM-CSF (4 ng/mL), and IL-4 (10 ng/mL) for one day at 37°C and 50 mL/L CO2. These SGC-7901 tumor cell lysate-pulsed (TP) DCs were used for vaccination after one day. B16 TP DCs were used as control.
To confirm that tumor-specific cytotoxic T lymphocytes (CTLs) can be generated ex vivo, splenic CD3+ T cells (1 × 106 cells/mL) were magnetically isolated from naïve BALB/c mice using CD3 microBeads. These cells were cultured in RPMI medium 1640 containing 10% FCS, then primed ex vivo in the presence of cytokines including IL-2 and IL-7 (5 ng/mL) at day 0, 7, and 14 with SGC-7901 TP DCs or B16 TP DCs at a 1:20 stimulator to responder cell ratio. Unpulsed DCs and SGC-7901 tumor lysates were used as controls. Fresh medium containing IL-2 and IL-7 was exchanged every 4 d. The primed T cells were effector cells; SGC-7901 or B16 tumor cells were target cells. On day 21, target cell suspension was added into 96 well plates, and effector cells were titrated to dilutions of target cells by serial dilutions (E-T mix, E:T, 1:1, 5:1, 10:1, 20:1, 50:1, 100:1). Supernatant from each well was collected after 20 h and cytolytic activity against target SGC-7901 tumor cells and B16 tumor cells was measured with a Cytotoxicity Detection Kit (LDH; Boehringer Mannheim, Mannheim, Germany). IFNγ production was determined with the IFNγ ELISA kit (Endogen, Woburn, MA, USA.) at a stimulator to responder cell ratio of 1:20.
Differences were evaluated using Statistical Package for Social Science 11.0 (SPSS11.0). Statistical analysis was performed using Student’s t-test. Statistical tests were two-sided. P < 0.05 were considered to be statistically significant.
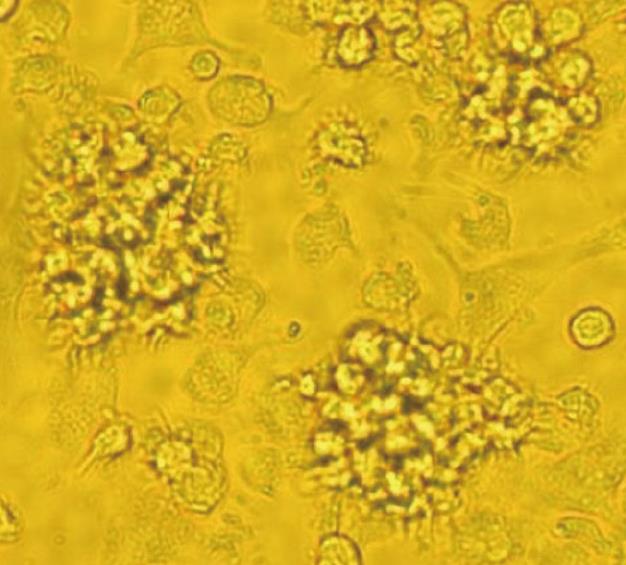
c-kit+ hematopoietic progenitor cells from the BALB/c mice bone marrow cells were cultured in presence of GM-CSF, IL-4, or TNFα for 8 d, then observed by light microscopy. Results show large cells with oval or irregularly shaped nuclei and many small dendrites (Figure 1).
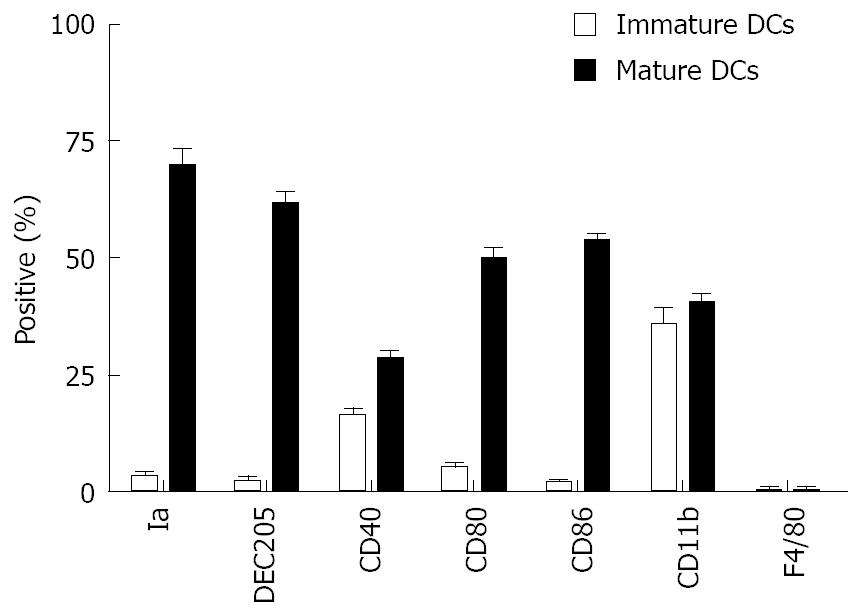
The phenotypic profile of a representative population of bone marrow DCs was determined by FACS. BM-DCs cultured for 5 d expressed moderate levels of co-stimulatory molecule CD40, high levels of CD11b, and very low levels of Ia, DEC-205, CD80, CD86, and were negative for F4/80 (Figure 2). However, when these cells were cultured in the presence of GM-CSF, IL-4, or TNFα for 8 d, they differentiated into mature DCs that expressed high levels of Ia, DEC-205, CD11b, CD80 and CD86 antigen, moderate levels of the co-stimulatory molecule CD40, and were negative for F4/80 (Figure 2).
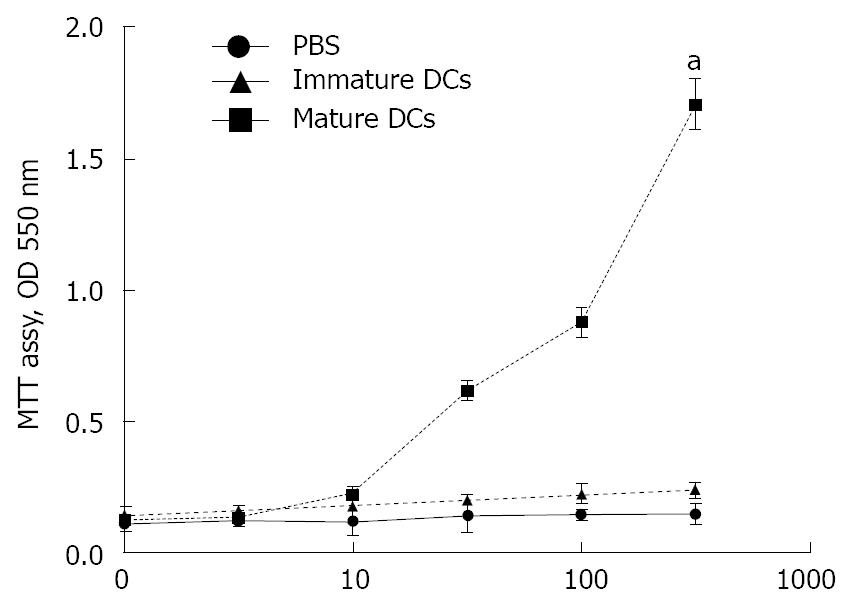
Allogeneic mixed-leukocyte reactions were performed using splenic T cells purified from B6 mice as responder cells. BM-DCs were treated with MMC to arrest cell proliferation and were used as stimulator cells. T cell proliferation was determined by using an MTT assay. Results show BM-derived mature DCs have the capacity to stimulate allogeneic T cells (Figure 3); however, BM-derived immature DCs and PBS did not simulate allogeneic T cells (Stimulator cells: 5 × 104 cells/mL, OD 550 nm: 1.74 ± 0.15 in mature DCs group versus 0.22 ± 0.05 in immature DCs group or 0.16 ± 0.04 in PBS group, P < 0.05, Figure 3).
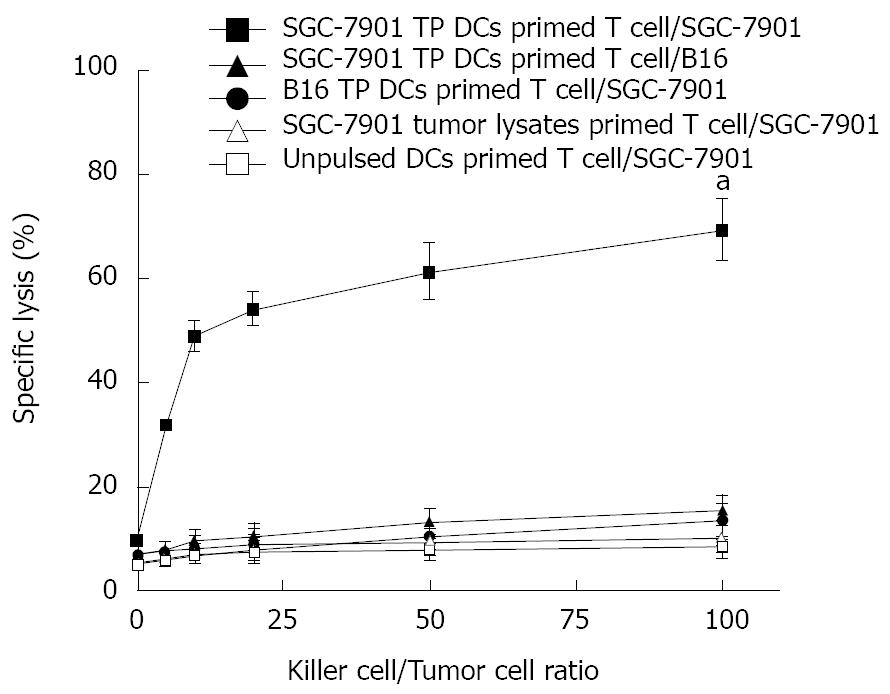
To study the potential of in anti-tumor immunity in vitro, BM-derived DCs were prepared by pulsing BM-DCs with tumor lysates after 5 d of culture in the presence of GM-CSF and IL-4. Naïve mouse splenic T cells were primed in vitro with SGC-7901 TP DCs or B16 TP DCs in the presence of IL-2 and IL-7 to elicit cytolytic reactivity against tumor cells. The results show that T cells in vitro primed with SGC-7901 TP DCs were able to lyse efficiently and specifically parental SGC-7901 tumor cells, but not B16 tumor cells, and T cells primed with B16 TP DCs, SGC-7901 tumor lysates or unpulsed DCs did not induce specific CTL against SGC-7901 tumor cells (E:T = 100:1, 69.55% ± 6.05% specific lysis in the SGC-7901 TP DCs primed T cell/SGC-7901 group versus 15.72% ± 2.9% specific lysis in the SGC-7901 TP DCs primed T cell/B16 group, 13.75% ± 3.14% specific lysis in the B16 TP DCs primed T cell/SGC-7901 group, 10.6% ± 3.01% specific lysis in the SGC-7901 tumor lysates primed T cell/SGC-7901 group, or 8.75% ± 2.15% specific lysis in the unpulsed DCs primed T cell/SGC-7901 group, P < 0.05, Figure 4).
Splenic CD3+ T cells primed with SGC-7901 TP DCs produced higher levels of IFNγin vitro when stimulated with SGC-7901 tumor cells; however, they did not produce higher levels of IFNγ when stimulated with B16 tumor cells (1575.31 ± 60.25 pg/mL in the SGC-7901 group versus 164.11 ± 18.52 pg/mL in the B16 group, P < 0.01, Figure 5).
In our experiments were obtained successfully from BALB/c mouse bone marrow precursors. These BM-DCs were analysed by morphological observation, phenotype analysis, and mixed lymphocyte reaction (MLR). This study demonstrates the generation of an effective CTL response against gastric carcinoma cells by repeated ex vivo stimulation of T cells with tumor lysate-pulsed DCs.
DCs induce, sustain and regulate immune responses[13]. Four stages of their development have been delineated: (1) bone marrow progenitors; (2) precursor DCs, which patrol through the blood, lymphatics and lymphoid tissues; (3) tissue-resident immature DCs, which possess high endocytic and phagocytic capacity permitting antigen (Ag)-capture; and (4) mature DCs, present within secondary lymphoid organs, expressing high levels of co-stimulatory molecules permitting Ag-presentation[14].
Immature DCs are characterized by high capacity for antigen uptake but low T-cell stimulatory capacity. DCs mature because DC-mediated immune responses are more effective if DCs receive an activation signal. This signal can be microbial products such as lipopolysaccharide or unmethylated CpG motifs mimicking bacterial DNA[15,16], inflammatory mediators such as TNFα and IL-6[17,18], or T cell-derived signals such as CD40 ligand[19]. Matured DCs up-regulate co-stimulatory molecules, secrete the T-cell differentiation factor IL-12[20], and present antigens more effectively because of increased phenotypic stability and extended half-life of MHC class I- and II-molecules[21]. Furthermore, immature DCs bear the danger of inducing non-proliferating, IL-10-producing T cells, whereas mature DCs promote the development of Th1 cells[22].
In our experiment, c-kit+ hematopoietic progenitor cells from mouse bone marrow cells cultured with GM-CSF, IL-4 or TNFα for 8 d showed the character of typical mature DCs. Morphologically, these large cells with oval or irregularly shaped nuclei and many small dendrites. Phenotypically, FACS analysis showed that they had typical mature DCs phenotypic markers, and expressed high levels of Ia, DEC-205, CD11b, CD80 and CD86 antigen, and moderate levels of CD40. Functionally, these cells gained the capacity to stimulate allogeneic T cells. However, immature DCs cultured with cytokines for 5 d did not possess typical DCs phenotypic markers and could not stimulate allogeneic T cells.
The use of a DC-based tumor vaccine as a cellular adjuvant to induce tumor-specific protective immunity holds great promise for cancer patients. This is important for advanced stage tumors with poor responsiveness to chemotherapy, such as gastric cancer. Currently, no defined tumor-specific antigen is available for many tumors, including gastric cancer. Now, DCs can be pulsed with synthetic peptides or proteins derived from known tumor associated antigens (TAA) such as MUC1, Her-2/neu, tyrosinase, CEA or Melan-A/MART[9].
In this study, tumor cell lysates were obtained by rapid freezing and thawing, and were regarded as tumor specific antigens. BM-derived DCs were pulsed with tumor lysates. Naïve mouse splenic T cells were primed in vitro with SGC-7901 TP DCs or B16 TP DCs to elicit cytolytic reactivity against tumor cells. The results showed that primed T cells in vitro with SGC-7901 TP DCs were able to induce specific CTL against SGC-7901 tumor cells, but not B16 tumor cells. Vaccination with DCs pulsed with tumor lysates has been shown to have efficient anti-tumor effects in many other tumor models and in clinical studies. Schnurr et al[23] report that T cells specific for pancreatic carcinoma cells can be generated in vitro by lysate-pulsed DCs and that the T-cell response can be enhanced by keyhole limpet hemocyanin (KLH). This in vitro model can be applied to compare different strategies in the development of DC-based tumor vaccines. Primary clinical studies were performed on melanoma patients using DCs pulsed with peptides or loaded with tumor cell lysates. Kono et al[24] report that tumor vaccination therapy with DCs pulsed with HER-2/neu-peptides may be a potential candidate for the novel treatment of gastric cancer patients. Nine gastric cancer patients with recurrent or unrescetable tumor were enrolled in the clinical trial. Vaccinations with DCs pulsed with HER-2 (p369) peptide were performed at 2-week intervals. In 3 of 9 patients, the tumor markers (CEA or CA19-9) were decreased after vaccination. Two patients had a tumor regression of more than 50%, and two presented a mixed response.
In summary, vaccination with bone marrow-derived dendritic cells pulsed with tumor cell lysates induced anti-tumor immunity specific to gastric cancer ex vivo. These results suggest that an evaluation of BM-DCs pulsed with tumor lysates against gastric cancer is an important next step in vivo. A trial evaluating this approach in mice is currently in preparation.
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that both initiate and modulate the immune response. DCs are cells in the pathway of antigen capture and presentation to T cells, with the unique ability to directly prime naïve CD4+ and CD8+ T cells. Gastric cancer is one of the most common cancers. Although gastric cancer therapy has made great progress, it is still difficult to treat advanced gastric cancer, as it has spread to the lymph glands and metastasized. Currently, tumor immunotherapy for gastric cancer has potential. DCs are believed to be essential for stimulating tumor-specific cytotoxic T lymphocyte (CTL) and inducing the protective and therapeutic anti-tumor immunity.
Currently, no defined tumor specific antigen is available for many tumors, including gastric cancer. Now, DCs can be pulsed with synthetic peptides or proteins derived from known tumor associated antigens (TAA) such as MUC1, Her-2/neu, tyrosinase, CEA or Melan-A/MART. Some studies have shown that it is feasible that DCs-based tumor vaccines will become a new effective immunoadjuvant therapy for gastric cancer, which can decrease the incidence and recurrence rate after operation for gastric cancer. Primary clinical studies were performed on melanoma patients using DCs pulsed with peptides or loaded with tumor cell lysates. Kono et al[24] report that tumor vaccination therapy with DCs pulsed with HER-2/neu-peptides may be a potential candidate for the novel treatment of gastric cancer patients.
c-kit+ hematopoietic progenitor cells from mice bone marrow cells cultured with GM-CSF, IL-4 or TNFα for 8 d showed the character of typical mature DCs. Gastric cancer cell lysates were obtained by rapid freezing and thawing, and were regarded as tumor specific antigens. Vaccination with bone marrow-derived dendritic cells pulsed with tumor cell lysates induced anti-tumor immunity specific to gastric cancer ex vivo.
Vaccination with bone marrow-derived dendritic cells pulsed with tumor cell lysates induced anti-tumor immunity specific to gastric cancer ex vivo. These results suggest that an evaluation of BM-DCs pulsed with tumor lysates against gastric cancer is an important next step in vivo. A trial evaluating this approach in mice is currently in preparation.
DCs means dendritic cells, BM-DCs indicates bone marrow DCs, APCs stands for antigen presenting cells, MHC is major histocompatibility complex, CTL is cytotoxic T lymphocyte, IFNγ means interferon gamma.
Using an in vitro system, the authors suggest a specific anti-tumor effect based on immunological mechanisms. The fatal prognosis of cancer requires the development of novel therapeutic concepts. Unfortunately, various promising strategies have failed in the clinical practice. Therefore, suitable animal models are strongly needed to investigate the possible effectiveness of the shown ex vivo principle for an anti-cancer treatment.
Peer reviewer: Gisela Sparmann, MD, Division of Gastroenterology, Department of Internal Medicine, University of Rostock, Ernst-Heydemann-Str. 6, Rostock D-18057, Germany
S- Editor Xiao LL L- Editor Li M E- Editor Yin DH
| 1. | Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195-1203. [Cited in This Article: ] |
| 2. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [Cited in This Article: ] |
| 4. | Guida F, Formisano G, Esposito D, Antonino A, Conte P, Bencivenga M, Persico M, Avallone U. [Gastric cancer: surgical treatment and prognostic score]. Minerva Chir. 2008;63:93-99. [Cited in This Article: ] |
| 5. | Liakakos T, Fatourou E. Stage-specific guided adjuvant treatment for gastric cancer. Ann Surg Oncol. 2008;15:2622-2623. [Cited in This Article: ] |
| 6. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103-108. [Cited in This Article: ] |
| 7. | Ishigami S, Natsugoe S, Uenosono Y, Hata Y, Nakajo A, Miyazono F, Matsumoto M, Hokita S, Aikou T. Infiltration of antitumor immunocytes into the sentinel node in gastric cancer. J Gastrointest Surg. 2003;7:735-739. [Cited in This Article: ] |
| 8. | Galetto A, Contarini M, Sapino A, Cassoni P, Consalvo E, Forno S, Pezzi C, Barnaba V, Mussa A, Matera L. Ex vivo host response to gastrointestinal cancer cells presented by autologous dendritic cells. J Surg Res. 2001;100:32-38. [Cited in This Article: ] |
| 9. | Wu Y, Wang L, Zhang Y. Dendritic cells as vectors for immunotherapy of tumor and its application for gastric cancer therapy. Cell Mol Immunol. 2004;1:351-356. [Cited in This Article: ] |
| 10. | Zhang Y, Harada A, Wang JB, Zhang YY, Hashimoto S, Naito M, Matsushima K. Bifurcated dendritic cell differentiation in vitro from murine lineage phenotype-negative c-kit+ bone marrow hematopoietic progenitor cells. Blood. 1998;92:118-128. [Cited in This Article: ] |
| 11. | Zhang Y, Zhang YY, Ogata M, Chen P, Harada A, Hashimoto S, Matsushima K. Transforming growth factor-beta1 polarizes murine hematopoietic progenitor cells to generate Langerhans cell-like dendritic cells through a monocyte/macrophage differentiation pathway. Blood. 1999;93:1208-1220. [Cited in This Article: ] |
| 12. | Zhang Y, Yoneyama H, Wang Y, Ishikawa S, Hashimoto S, Gao JL, Murphy P, Matsushima K. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1alpha in mice. J Natl Cancer Inst. 2004;96:201-209. [Cited in This Article: ] |
| 13. | Ullrich E, Ménard C, Flament C, Terme M, Mignot G, Bonmort M, Plumas J, Chaperot L, Chaput N, Zitvogel L. Dendritic cells and innate defense against tumor cells. Cytokine Growth Factor Rev. 2008;19:79-92. [Cited in This Article: ] |
| 14. | Nouri-Shirazi M, Banchereau J, Fay J, Palucka K. Dendritic cell based tumor vaccines. Immunol Lett. 2000;74:5-10. [Cited in This Article: ] |
| 15. | Liu Q, Shu X, Sun A, Sun Q, Zhang C, An H, Liu J, Cao X. Plant-derived small molecule albaconol suppresses LPS-triggered proinflammatory cytokine production and antigen presentation of dendritic cells by impairing NF-kappaB activation. Int Immunopharmacol. 2008;8:1103-1111. [Cited in This Article: ] |
| 16. | Warren TL, Bhatia SK, Acosta AM, Dahle CE, Ratliff TL, Krieg AM, Weiner GJ. APC stimulated by CpG oligodeoxynucleotide enhance activation of MHC class I-restricted T cells. J Immunol. 2000;165:6244-6251. [Cited in This Article: ] |
| 17. | Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, Rothenfusser S, Endres S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633-1641. [Cited in This Article: ] |
| 18. | Su B, Wang J, Wang X, Jin H, Zhao G, Ding Z, Kang Y, Wang B. The effects of IL-6 and TNF-alpha as molecular adjuvants on immune responses to FMDV and maturation of dendritic cells by DNA vaccination. Vaccine. 2008;26:5111-5122. [Cited in This Article: ] |
| 19. | Gonzalez-Carmona MA, Lukacs-Kornek V, Timmerman A, Shabani S, Kornek M, Vogt A, Yildiz Y, Sievers E, Schmidt-Wolf IG, Caselmann WH. CD40ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology. 2008;48:157-168. [Cited in This Article: ] |
| 20. | He XZ, Wang L, Zhang YY. An effective vaccine against colon cancer in mice: use of recombinant adenovirus interleukin-12 transduced dendritic cells. World J Gastroenterol. 2008;14:532-540. [Cited in This Article: ] |
| 21. | Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, Ploegh HL. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983-988. [Cited in This Article: ] |
| 22. | Jalili A. Dendritic cells and their role in cancer immuno-therapy. Iran J Immunol. 2007;4:127-144. [Cited in This Article: ] |
| 23. | Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S, Eigler A. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001;61:6445-6450. [Cited in This Article: ] |
| 24. | Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394-3400. [Cited in This Article: ] |