Published online Dec 28, 2008. doi: 10.3748/wjg.14.7386
Revised: November 25, 2008
Accepted: December 2, 2008
Published online: December 28, 2008
AIM: To discuss the expression of glactin-3 in liver metastasis of colon cancer and its inhibition by modified citrus pectin (MCP) in mice.
METHODS: Seventy-five Balb/c mice were randomly divided into negative control group (n = 15), positive control group (n = 15), low MCP concentration group (n = 15), middle MCP concentration group (n = 15) and high MCP concentration group (n = 15). CT26 colon cancer cells were injected into the subcapsule of mouse spleen in positive control group, low, middle and high MCP concentrations groups, except in negative control, to set up a colon cancer liver metastasis model. The concentration of MCP in drinking water was 0.0%, 0.0%, 1.0%, 2.5% and 5.0% (wt/vol), respectively. Liver metastasis of colon cancer was observed after 3 wk. Enzyme-linked immunosorbent assay (ELISA) was used to detect the concentration of galectin-3 in serum. Expression of galectin-3 in liver metastasis was detected by immunohistochemistry.
RESULTS: Except for the negative group, the percentage of liver metastasis in the other 4 groups was 100%, 80%, 73.3% and 60%, respectively. The number of liver metastases in high MCP concentration group was significantly less than that in positive control group (P = 0.008). Except for the negative group, the median volume of implanted spleen tumor in the other 4 groups was 1.51 cm3, 0.93 cm3, 0.77 cm3 and 0.70 cm3, respectively. The volume of implanted tumor in middle and high MCP concentration groups was significantly smaller than that in positive control group (P = 0.019; P = 0.003). The concentration of serum galectin-3 in positive control and MCP treatment groups was significantly higher than that in the negative control group. However, there was no significant difference between them. Except for the negative control group, the expression of galectin-3 in liver metastases of the other 4 groups showed no significant difference.
CONCLUSION: Expression of galectin-3 increases significantly in liver metastasis of colon cancer, which can be effectively inhibited by MCP.
- Citation: Liu HY, Huang ZL, Yang GH, Lu WQ, Yu NR. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J Gastroenterol 2008; 14(48): 7386-7391
- URL: https://www.wjgnet.com/1007-9327/full/v14/i48/7386.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.7386
| Groups | n | Numbers of liver metastases | χ2 | P | |||
| 0 (0) | I (1-5) | II (6-10) | III (> 10) | ||||
| Positive control group | 15 | 0 | 2 | 7 | 6 | ||
| 1.0% MCP group | 15 | 3 | 6 | 2 | 4 | 3.996 | > 0.05 |
| 2.5% MCP group | 15 | 4 | 7 | 1 | 3 | 7.069 | > 0.05 |
| 5.0% MCP group | 15 | 6 | 4 | 2 | 3 | 8.052 | < 0.051 |
| Groups | n | mean ± SD | H | P |
| Negative control group | 15 | 14.63 ± 10.08 | 9.37 | < 0.011 |
| Positive control group | 15 | 91.01 ± 12.94 | 4.34 | |
| 1.0% MCP group | 15 | 82.75 ± 20.33 | ||
| 2.5% MCP group | 15 | 79.01 ± 17.64 | ||
| 5.0% MCP group | 15 | 85.94 ± 15.52 |
| Groups | n | Expression of galectin-3 | H | P | |||
| (-) | (+) | (++) | (+++) | ||||
| Positive control group | 15 | 5 | 6 | 2 | 2 | 0.52 | P = 0.170 |
| 1.0% MCP group | 12 | 4 | 3 | 2 | 3 | 0.52 | P = 0.170 |
| 2.5% MCP group | 11 | 2 | 6 | 2 | 1 | 0.52 | P = 0.170 |
| 5.0% MCP group | 9 | 3 | 4 | 1 | 1 | 0.52 | P = 0.170 |
Liver metastasis is the main cause that impacts the therapeutic effect and postoperative prognosis of colorectal cancer. Inhibiting liver metastasis is beneficial to the therapeutic effect and postoperative prognosis of colorectal cancer[1]. Galectin-3, a carbohydrate-binding protein on tumor cell surface, is closely related to cell to cell adhesion, aggregation of cancer cells in vitro, tumor growth and metastasis in vivo[2,3]. Galectin-3 is highly expressed in a variety of metastatic cancer cells[4]. Galactosyl, a main component of modified citrus pectin (MCP), can specifically inhibit tumor growth and metastasis in vivo and galectin-3-mediated functions in vitro[5]. Few studies are available dealing with the inhibitory effects of MCP on cancer metastasis. The aim of this study was to discuss the inhibitory effect of MCP on liver metastasis in a rat colon cancer model.
Mouse colon adenocarcinoma cell line (CT-26), preserved and passaged in our biotechnology laboratory, was cultivated in RPMI-1640 culture medium containing 10% new born calf serum, penicillin G and streptomycin at 37°C in an 5% CO2 incubator containing 50 mL/L CO2.
Seventy-five 6-8 wk old Balb/c female mice, offered by Guangdong Medical Laboratory Animal Center (certification No. 2006A019), weighing 20-25 g, were used in this study. The mice were free from specified-pathogens. Experiments were performed in the SPF Animal Laboratory.
MCP was provided by Centraxinc International, Inc (Francisco, USA). Mouse galectin-3 ELISA kit was provided by R&D Company (Minneapolis, USA). Mouse galectin-3 affinity purified pol was purchased from Jingmei Biotech Co, Lid (Shanghai, China).
U.S Beecher tissue microarray meter, ST360 auto ELIASA were purchased from Kehua (Shanghai, China).
Seventy-five Balb/c mice were randomly divided into negative control group, positive control group, low MCP concentration group, middle MCP concentration group and high MCP concentration group. The concentration of MCP in drinking water was 0.0%, 0.0%, 1.0%, 2.5% and 5.0% (wt/voL), respectively. CT26 cells in exponential growth with sufficient NS were used to mix up into a suspension (1 × 106/mL). The mice were anesthetized with 4% chloral hydrate (10 mL/kg) by injecting into their abdominal cavity and an abdominal wall incision paralleling the left subcostal margin was then made. Laparotomy was performed and 0.05 mL of CT-26 suspension was injected into the spleen. A same volume of NS was injected into the abdominal cavity of mice in the negative control group. The incision was closed with #1 suture. All mice continuously received MCP dissolved in drinking water from the 2nd d after operation, to the necropsy day 21. A same volume of distilled water was given in negative control group. All mice had free access to food and water during the experiment.
After a 3-wk observation, the eyeball of mice was removed to collect 0.5-1.0 mL peripheral blood. All mice were killed by decapitation. The abdominal cavity was opened to observe primary neoplasms of the spleen and record the volume and number of neoplasms (volume = ab2/2, a = max diameter, b = min diameter). The total volume was recorded if there were more than 2 neoplasms. The number of liver metastases was calculated. All neoplasms were identified with HE staining. Liver metastasis was divided into 4 grades as previously described[6]: grade 0: no liver metastases; gradeI: 1-5 liver metastases; grade II: 6-10 liver metastases; grade III: more than 10 liver metastases.
Blood sample was centrifuged at 3000 r/min for 5 min to separate serum. The isolated serum was stored at ≤ -20°C. The serum sample was diluted in a diluent at 1: 20. In brief, 100 μL of a diluent was added into each well of a plate and incubated for 2 h at room temperature, and the plate was washed with a washing buffer. One hundred μL of detection antibody was added into each well of a plate, incubated for 2 h at room temperature, and the plate was washed with a washing buffer. One hundred μL of a working diluent of streptavidin-HRP was added into each well of a plate, incubated for 20 min at room temperature in the dark, the plate was washed. Finally, 100 μL of a substrate solution was added into each well of a plate, incubated for 20 min at room temperature in the dark, and 50 μL of a stop solution was then added into each well of the plate. A microplate reader was used to read the absorbance at 450 nm, then a standard curve was plotted and a formula was used to fit the OD of standard samples.
Liver tissue sections were stained with HE to select typical nidi, such as a region rich of neoplasms but lack of necrosed areas and bleeding. A tissue microarray meter was used to perforate into a paraffin block (25 mm × 25 mm × 20 mm). The diameter of each hole was 1.2 mm, and the distance between two holes was 1.0 mm. Fifty holes were arranged in 10 lines and 5 arrays. A 1.2-mm long puncture needle was used to draw out the marked typical tissue core and to transfer it to a certain location on the paraffin block. Forty-seven metastasis samples were arranged into 2 paraffin blocks. Each sample included 2 marked cores. The tissue array paraffin was kept on a 55°C copper board for 30 min. The paraffin block was pressed gently to array the tissue cores and cooled at room temperature. The arrays were sliced quickly after pre-cooled at 4°C for 4 h.
The tissue sample sections were stained with galectin-3 immunohistochemistry following the instructions provided with galectin-3 affinity purified polyclonal antibody. The sections were deparaffined and hydrated. After washed with PBS, the sections were incubated with 3% hydrogen dioxide for 10 min at room temperature, with antibody for 10 min at room temperature, with EnVision for 30 min at room temperature, finally with DAB for color development. The results were judged double-blindly by 2 pathologists. The level of galectin-3 expression was classified into negative (-), weakly positive (+), positive (++) and strong positive (+++) as previously described[7].
All the data were analyzed by SPSS10.0 Software. Tumor volume, number of liver metastases, concentration of galectin-3 in serum and tissue were analyzed by non-parametric test.
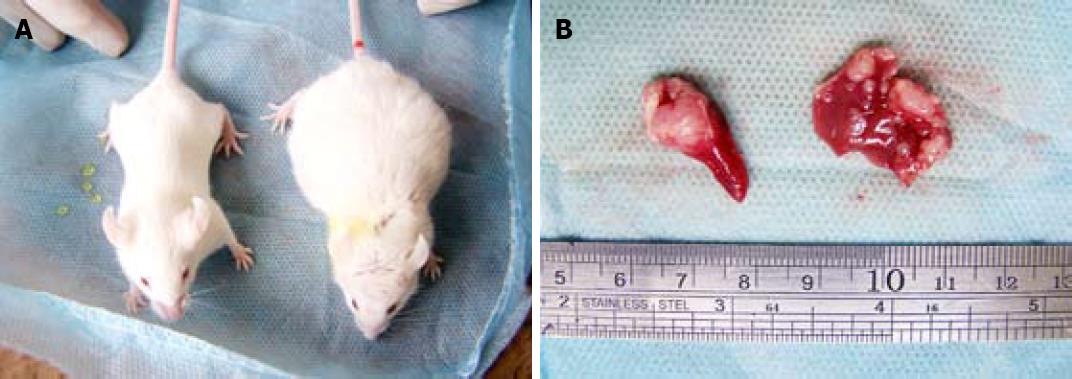
No mouse died during the 3-wk experiment period. Some mice were found to have tumor mass bulging on the abdominal wall. Some of the cancer-carrying mice appeared signs of mental depression, such as reduced activity, slow response, gloomy hair color, loss of appetite (Figure 1).
Except for the negative control group, the liver metastatic rate for the other 4 groups treated with high, middle and low MCP concentrations was 100%, 80%, 73.3% and 60%, respectively. The number of liver metastases in high MCP concentration group was significantly less than that in low and middle MCP concentration groups (P < 0.05) (Table 1).
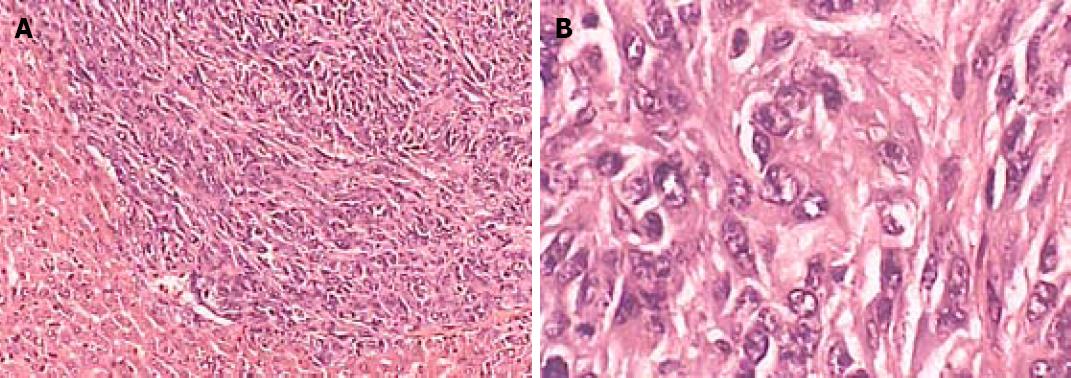
The median volume of implanted spleen tumor in high, middle and low MCP concentration groups was 1.51 cm3, 0.93 cm3, 0.77 cm3 and 0.70 cm3, respectively. No tumor was found in negative control group. The volume of tumor in high MCP concentration group was significantly lower than that in middle and low MCP concentration groups (P < 0.05) (Table 2, Figure 2).
The concentration of galectin-3 in serum samples calculated according to the standard regression formula was (14.63 ± 10.08) ng/mL in negative control group, (91.01 ± 22.94) ng/mL in positive control group, (82.75 ± 20.33) ng/mL in low MCP concentration group, (79.01 ± 17.64) ng/mL in middle MCP concentration group and (85.94 ± 15.52) ng/mL in high MCP concentration group, respectively. The results indicate that the concentration of serum galectin-3 in positive control group and MCP treatment groups was significantly higher than that in negative control group (P < 0.01, Table 3).
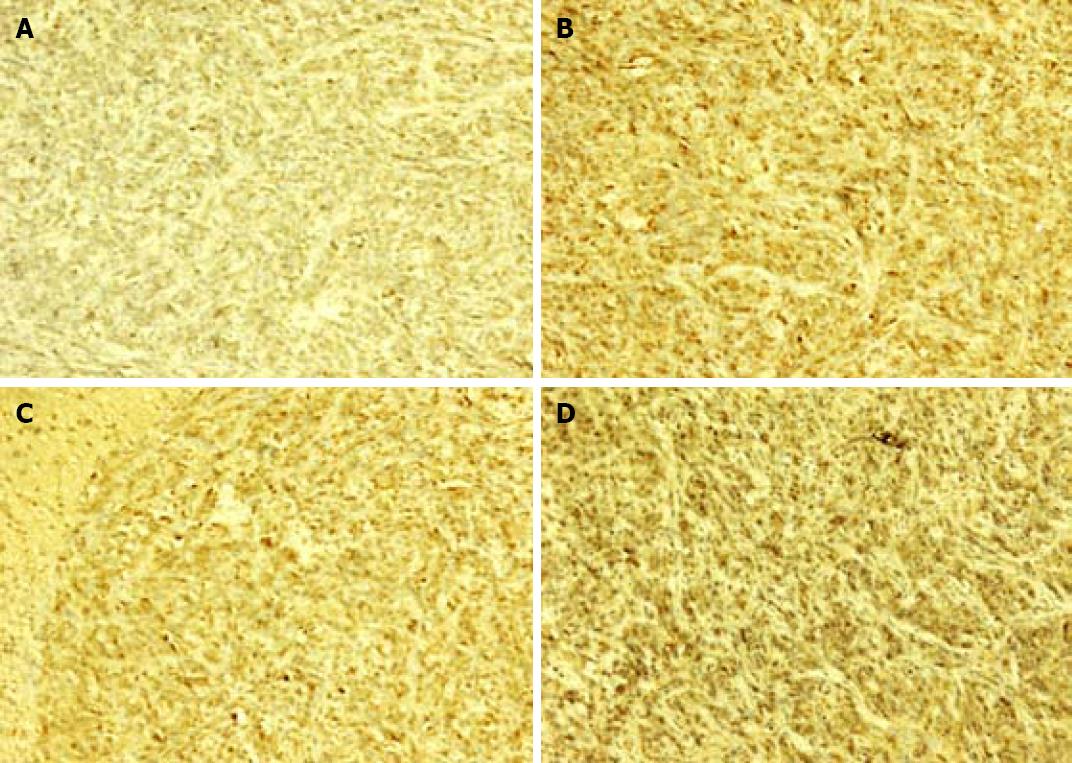
Brown cells in cytolymph under microscope were considered positive cells. The percentage of positive cells in metastatic liver tissue showed that galectin-3 had no significant difference in liver metastases positive control and MCP treatment groups (Figure 3, Table 4).
Liver metastasis of colon cancer includes tumor cell infiltration, exfoliation, adhesion, aggregation and invasion, which involve carbohydrate-mediated recognition proteins, such as the galectins. Adhesion of tumor cells to tumor embolus and anchorage of tumor cells to blood vessel endothelium or basement membrane are the two crucial steps of liver metastasis of colon cancer. Different galectins expressed in different steps of metastasis cascade might play a crucial role in tumor progression[8]. Galectin-3, a member of the lectin family, is a multifunctional oncogenic protein which regulates cell growth, adhesion[9], proliferation and apoptosis, as well as cell-cell interaction and angiogenesis[10-13]. A large body of evidence has confirmed that metastatic cancer cells significantly express galectin-3, and high expression of galectin-3 can be detected in both primary and metastatic lesions[14], even in blood[15], showing a strong relation with cancer growth and metastasis[16-18]. Moreover, the expression of galectin-3 can be used as a diagnostic and prognostic marker of colorectal cancer[19-21]. Therefore, if the function of galectin-3 is blocked, the progression of adhesion and aggregation can be intercepted, which may stimulate the development of novel drugs for the targeted treatment of colorectal cancer and other cancers[22].
MCP is a non-digestible, water-soluble polysaccharide fiber derived from citrus fruits, and also a complex polysaccharide rich in galactosyl residues. MCP can specifically inhibit carbohydrate-binding protein as a high affinitive ligand[23]. When the concentration of MCP reaches an adequate level, galectin-3 protein on the surface of cancer cells would be almost completely blocked by MCP molecules. As a result, the procession of adhesion and aggregation between cancer cells will be intercepted. In addition, MCP can inhibit morphogenesis of endothelial cells and angiogenesis by blocking galectin-3, thus intercepting cancer cells to absorb nutrition from vessels and cancer progression[24,25]. However, there is no evidence that MCP attacks cancer cells directly or indirectly with or without toxicity and side effects[26]. In vitro experiments have shown that MCP is able to inhibit adhesion of cancer cells to laminin and homotypic aggregation[27]. Animal experiments also showed that oral MCP can inhibit the growth and metastases of rat prostate cancer cells[28], human breast cancer[5] and melanoma cells[29-31].
The results of our study show that MCP could effetely inhibit the growth and metastasis of implanted colon cancer in mouse spleen. The number of liver metastases and tumor volume in high MCP concentration group were significantly less and smaller than those in control group, indicating that MCP can inhibit the growth and metastasis of colon cancer in a dose-dependent manner, which is consistent with the reported data[5,28-30]. In contrast, low MCP concentration group showed no significant difference in colon cancer growth and liver metastasis, which may be due to the lack of samples and the low sensitivity of non-parametric statistics. Further studies are needed to clarify the role of MCP concentration in this regard.
ELISA and immunohistochemistry analysis have shown that MCP does not impact glactin-3 concentration and expression in liver metastatic cancer cells, but inhibits liver metastasis in vitro[30]. The possible mechanism is that MCP only blocks out galectin-3 molecules on the surface of cancer cells, but does not intercept the expression or secretion of cancer cells. It was recently reported that galectin-3 can be used as a reliable diagnostic marker of colorectal cancer and is one of the target proteins in cancer treatment[22].
In conclusion, MCP can effectively inhibit the growth of colon cancer and liver metastasis by intercepting the adhesion and aggregation of cancer cells. MCP, as a natural polysaccharide derived from fruits and a nontoxic drug, may pave a new way in controlling the growth and metastasis of colon cancer and other cancers. The role of MCP and chemotherapy in controlling and curing liver metastatic colon cancer needs further study.
Galectin-3 is a carbohydrate-binding protein closely related with cancer growth and metastasis. Studies have shown that galectin-3 is over-expressed in different types of cancer. Dietary components play an important role in cancer progression and metastasis, carbohydrate-mediated recognition processes participate in cancer progression. Modified citrus pectin (MCP), a non-digestible and water-soluble polysaccharide fiber derived from citrus fruits, can inhibit galectin-3-mediated function in vivo and in vitro.
The role of galectin-3 in cancer growth and metastasis is an important field in tumor research. Several experimental studies have reported the specific inhibitory effect of MCP on different cancer cells in xenograft models. The present study investigated the effect of MCP on colon cancer growth and liver metastasis in vivo. In addition, the expression of galectin-3 was also studied to describe the possible anticancer mechanism of MCP.
Few experiments have been conducted to observe the effect of MCP on preventing cancer growth and metastasis in vivo. This study showed the inhibitory effect of MCP on liver metastasis of colon cancer in a mouse model. Besides, the expression of galectin-3 in tumor tissue and serum was tested to describe the galectin-3 status during MCP treatment.
This study demonstrated that galectin-3 was over-expressed in liver metastasis of colon cancer and oral MC could inhibit cancer growth and metastasis in a mouse model. The results show that galectin-3, as a potential marker and therapeutic target of colorectal cancer, played an important role in prevention and treatment of cancer.
Citrus pectin is a complex polysaccharide fiber derived from the pulp and peel of citrus fruits. Citrus pectin is rich in galactosyl, a ligand for galectin-3, when it is modified by high-pH and temperature. MCP inhibits galectin-3 function when citrus fruits are combined with galectin-3.
In this manuscript, the authors studied the inhibitory effect of MCP on liver metastasis in a mouse colon cancer model. The study demonstrated that MCP inhibited liver metastasis by suppressing the function of galectin-3. The study is well designed and the results are reliable.
Peer reviewer: Xin-Yuan Guan, Professor, Department of Clinical Oncology, The University of Hong Kong, Room L10-56, 10/F, Laboratory Block, 21 Sassoon Road, Pokfulam, Hong Kong, China
S- Editor Tian L L- Editor Wang XL E- Editor Ma WH
| 1. | Kindler HL, Shulman KL. Metastatic colorectal cancer. Curr Treat Options Oncol. 2001;2:459-471. [Cited in This Article: ] |
| 2. | Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616-635. [Cited in This Article: ] |
| 3. | Krzeslak A, Lipinska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004;9:305-328. [Cited in This Article: ] |
| 4. | Califice S, Castronovo V, Van Den Brole F. Galectin-3 and cancer (Review). Int J Oncol. 2004;25:983-992. [Cited in This Article: ] |
| 5. | Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854-1862. [Cited in This Article: ] |
| 6. | Zhou ZW, Wan DS, Wang GQ, Ren JQ, Lu ZH, Lin SX, Tang SX, Ye YL, Chen G. [Inhibitory effect of angiogenesis inhibitor YH-16 on liver metastases from colorectal cancer]. Ai Zheng. 2006;25:818-822. [Cited in This Article: ] |
| 7. | Sanjuan X, Fernandez PL, Castells A, Castronovo V, van den Brule F, Liu FT, Cardesa A, Campo E. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology. 1997;113:1906-1915. [Cited in This Article: ] |
| 8. | Grassadonia A, Tinari N, Iurisci I, Piccolo E, Cumashi A, Innominato P, D’Egidio M, Natoli C, Piantelli M, Iacobelli S. 90K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj J. 2004;19:551-556. [Cited in This Article: ] |
| 9. | Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667-676. [Cited in This Article: ] |
| 10. | Nakahara S, Raz A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605-610. [Cited in This Article: ] |
| 11. | Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007;10:101-108. [Cited in This Article: ] |
| 12. | Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004;19:543-549. [Cited in This Article: ] |
| 13. | Zou J, Glinsky VV, Landon LA, Matthews L, Deutscher SL. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis. 2005;26:309-318. [Cited in This Article: ] |
| 14. | Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389-1393. [Cited in This Article: ] |
| 15. | Greco C, Vona R, Cosimelli M, Matarrese P, Straface E, Scordati P, Giannarelli D, Casale V, Assisi D, Mottolese M. Cell surface overexpression of galectin-3 and the presence of its ligand 90k in the blood plasma as determinants in colon neoplastic lesions. Glycobiology. 2004;14:783-792. [Cited in This Article: ] |
| 16. | Bresalier RS, Mazurek N, Sternberg LR, Byrd JC, Yunker CK, Nangia-Makker P, Raz A. Metastasis of human colon cancer is altered by modifying expression of the beta-galactoside-binding protein galectin 3. Gastroenterology. 1998;115:287-296. [Cited in This Article: ] |
| 17. | Tsuboi K, Shimura T, Masuda N, Ide M, Tsutsumi S, Yamaguchi S, Asao T, Kuwano H. Galectin-3 expression in colorectal cancer: relation to invasion and metastasis. Anticancer Res. 2007;27:2289-2296. [Cited in This Article: ] |
| 18. | Zhang N, Ding YQ, Liang L. [Association of galectin-3 expression with biological behaviors of human colorectal carcinoma]. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:1685-1689. [Cited in This Article: ] |
| 19. | Endo K, Kohnoe S, Tsujita E, Watanabe A, Nakashima H, Baba H, Maehara Y. Galectin-3 expression is a potent prognostic marker in colorectal cancer. Anticancer Res. 2005;25:3117-3121. [Cited in This Article: ] |
| 20. | Bresalier RS, Byrd JC, Tessler D, Lebel J, Koomen J, Hawke D, Half E, Liu KF, Mazurek N. A circulating ligand for galectin-3 is a haptoglobin-related glycoprotein elevated in individuals with colon cancer. Gastroenterology. 2004;127:741-748. [Cited in This Article: ] |
| 21. | Nakamura M, Inufusa H, Adachi T, Aga M, Kurimoto M, Nakatani Y, Wakano T, Nakajima A, Hida JI, Miyake M. Involvement of galectin-3 expression in colorectal cancer progression and metastasis. Int J Oncol. 1999;15:143-148. [Cited in This Article: ] |
| 22. | Shi Y, He B, Kuchenbecker KM, You L, Xu Z, Mikami I, Yagui-Beltran A, Clement G, Lin YC, Okamoto J. Inhibition of Wnt-2 and galectin-3 synergistically destabilizes beta-catenin and induces apoptosis in human colorectal cancer cells. Int J Cancer. 2007;121:1175-1181. [Cited in This Article: ] |
| 23. | Modified citrus pectin-monograph. Altern Med Rev. 2000;5:573-575. [Cited in This Article: ] |
| 24. | Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899-909. [Cited in This Article: ] |
| 25. | Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29-41. [Cited in This Article: ] |
| 26. | Chen CH, Sheu MT, Chen TF, Wang YC, Hou WC, Liu DZ, Chung TC, Liang YC. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem Pharmacol. 2006;72:1001-1009. [Cited in This Article: ] |
| 27. | Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J. 1994;11:527-532. [Cited in This Article: ] |
| 28. | Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J, Donat TL, Tait L, Hogan V, Raz A. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst. 1995;87:348-353. [Cited in This Article: ] |
| 29. | Hayashi A, Gillen AC, Lott JR. Effects of daily oral administration of quercetin chalcone and modified citrus pectin on implanted colon-25 tumor growth in Balb-c mice. Altern Med Rev. 2000;5:546-552. [Cited in This Article: ] |
| 30. | Platt D, Raz A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J Natl Cancer Inst. 1992;84:438-442. [Cited in This Article: ] |