Published online Jan 14, 2009. doi: 10.3748/wjg.15.226
Revised: October 29, 2008
Accepted: November 5, 2008
Published online: January 14, 2009
AIM: To study adherence to the widely accepted surveillance guidelines for patients with long-standing colitis in the Netherlands.
METHODS: A questionnaire was sent to all 244 gastroenterologists in the Netherlands.
RESULTS: The response rate was 63%. Of all gastroenterologists, 95% performed endoscopic surveillance in ulcerative colitis (UC) patients and 65% in patients with Crohn’s colitis. The American Gastroenterological Association (AGA) guidelines were followed by 27%, while 27% and 46% followed their local hospital protocol or no specific protocol, respectively. The surveillance was correctly initiated in cases of pancolitis by 53%, and in cases of left-sided colitis by 44% of the gastroenterologists. Although guidelines recommend 4 biopsies every 10 cm, less than 30 biopsies per colonoscopy were taken by 73% of the responders. Only 31%, 68% and 58% of the gastroenterologists referred patients for colectomy when low-grade dysplasia, high-grade dysplasia (HGD) or Dysplasia Associated Lesion or Mass (DALM) was present, respectively.
CONCLUSION: Most Dutch gastroenterologists perform endoscopic surveillance without following international recommended guidelines. This practice potentially leads to a decreased sensitivity for dysplasia, rendering screening for colorectal cancer in this population highly ineffective.
- Citation: van Rijn AF, Fockens P, Siersema PD, Oldenburg B. Adherence to surveillance guidelines for dysplasia and colorectal carcinoma in ulcerative and Crohn’s colitis patients in the Netherlands. World J Gastroenterol 2009; 15(2): 226-230
- URL: https://www.wjgnet.com/1007-9327/full/v15/i2/226.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.226
Patients with longstanding ulcerative colitis (UC) and Crohn’s disease have an increased risk of developing colorectal cancer. This severe complication of inflammatory bowel disease (IBD) generally develops in longstanding disease. If colorectal cancer has developed in patients with IBD, the mortality rate is higher than in patients with sporadic colorectal cancer[12]. The lifetime prevalence of colorectal carcinoma (CRC) in UC patients is estimated to be 2% after 10 years, 8% after 20 years, and even 18% after 30 years of extensive disease[3].
Surveillance of IBD for colorectal cancer is widely practiced, and is recommended by the American Gastroenterological Association (AGA), and the British Society of Gastroenterology (BSG) guidelines[4–6]. These guidelines aim to detect dysplasia or surgically curable cancer, and are thought to improve the prognosis. However, the reduction in mortality in patients with IBD and colorectal cancer through surveillance has still to be proven in large prospective randomized controlled trials. Table 1 gives an overview of the key elements in screening patients with long-standing, extensive IBD as recommended by the AGA[6]. These recommendations are applicable to both UC and Crohn’s colitis. Since dysplastic lesions in these patients often present as flat or depressed abnormalities, surveillance colonoscopies should be performed in combination with an extensive biopsy protocol. High-grade dysplasia (HGD) in flat mucosa or a Dysplasia Associated Lesion or Mass (DALM) is considered an indication for colectomy when the pathological findings are confirmed by a second experienced pathologist. There is still no consensus on management in cases of unifocal or multifocal low grade dysplasia (LGD) in flat mucosa. What complicates the issue, as earlier studies have indicated, is that there seems to be difficulty in confirming dysplasia by the pathologist[78]. The management of the different forms of dysplasia varies from no management or intensifying the screening program to immediate colectomy. When advising a patient on colectomy, other factors like age, a coexisting diagnosis of primary sclerosing cholangitis (PSC) or a family history of colorectal cancer should be taken into account.
| Key element | |
| Surveillance colonoscopy | Colonoscopy with systematic biopsies |
| Perform surveillance every 1 to 2 yr | |
| After 8 to 10 yr of disease in those with pancolitis | |
| After 15 yr of disease in those with left-sided colitis | |
| Biopsy protocol | Biopsies every 10 cm in all 4 quadrants. |
| Additional biopsies of strictures and mass lesions other than pseudopolyps | |
| Polyps that appear potentially dysplastic remove by polypectomy with biopsy of adjacent flat mucosa | |
| Dysplasia | If HGD or multifocal low-grade dysplasia is found in flat mucosa refer for colectomy |
| Presence of low-grade dysplasia, particularly if it is unifocal: no consensus | |
| DALM is an indication for colectomy | |
| Other factors of consideration to advise on colectomy | Ongoing colitis-related symptoms |
| Life expectancy | |
| Duration, severity and extent of colitis | |
| A personal history of primary sclerosing cholangitis | |
| A family history of colorectal cancer | |
| Discussion around the time of surveillance of benefit, harms, and short comings of colonoscopy surveillance |
As there are no current Dutch guidelines available regarding surveillance of IBD, we presumed that Dutch Gastroenterologists (GEs) would adopt the current AGA guidelines or other relevant guidelines. To investigate the effect of surveillance guidelines on the detection of dysplasia or colorectal cancer, the first step is to study adherence to these guidelines in clinical practice. This study was designed to assess whether screening programs and recommendations set by e.g. the AGA are used by Dutch GEs for patients with ulcerative or Crohn’s colitis and whether the guidelines are followed correctly.
After reviewing the widely used guidelines of the AGA, the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, in addition to the relevant literature, a questionnaire was developed. The questionnaire was focussed on the use, feasibility and ability to follow the screening guidelines, and contained 18 multiple choice questions and one open question. We asked the invited GEs if they practised surveillance in IBD patients, if they used one of the recommended guidelines and, if not, the reason why not. The other questions, all of a multiple choice design, were divided in four subgroups: (1) start of surveillance; (2) time interval between surveillance endoscopies; (3) biopsy protocol; and (4) management of dysplasia.
In the Netherlands, surveillance endoscopies are usually performed by gastroenterologists. As almost all gastroenterologists are also registered as members of the Dutch Gastroenterology Association, we only included registered gastroenterologists, with the exception of gastroenterologists that were still in training or did not work in Dutch hospitals (n = 34). To ensure the reliability of the answers provided, the questionnaire was anonymous, and to guarantee privacy of the hospitals involved, no questions were asked on the type of hospital (e.g. teaching, non-teaching). The questionnaire could be completed in less than 5 min. To increase the response rate, a reminder was sent to all GEs after 3 wk. Results were tabulated after the second letter. Data were statistically analysed using the Statistical Package for the Social Sciences or SPSS (version 12.0.2) using frequencies.
Of the 244 questionnaires, 153 were returned, yielding an overall response rate of 63%. Five GEs were excluded from further analysis: 2 were recently retired, 1 was currently working in another country and 2 stated they had no experience with IBD patients. The remaining 148 were analysed (61%).
Seven (5%) GEs did not provide surveillance for their IBD patients. Four GEs indicated that they would only include IBD patients in a surveillance program in cases with a positive family history of CRC, while 2 considered the available evidence in the literature to be insufficient to justify screening in this patient category. One GE did not explain his or her motivation.
Of all responding GEs, 95% (n = 141) provided surveillance for their IBD patients. Of these GEs, 46% stated that they did not follow any of the recommended guidelines, 27% followed the AGA guidelines, and 27% used a local protocol. Only 2 GEs followed the British guidelines. All GEs performed surveillance in UC patients, and 65% performed surveillance in patients with Crohn’s colitis.
All further results are based on the 95% (n = 141) of GEs who performed surveillance on IBD patients.
The start of surveillance depends on which time point is taken as the starting point, i.e. the diagnosis of IBD. This can be crucial as there can be a substantial delay between the onset of symptoms and diagnosis of IBD. Sixty nine percent started surveillance from the moment a firm diagnosis of Crohn’s disease or UC was made, whilst 31% started surveillance from the onset of IBD symptoms.
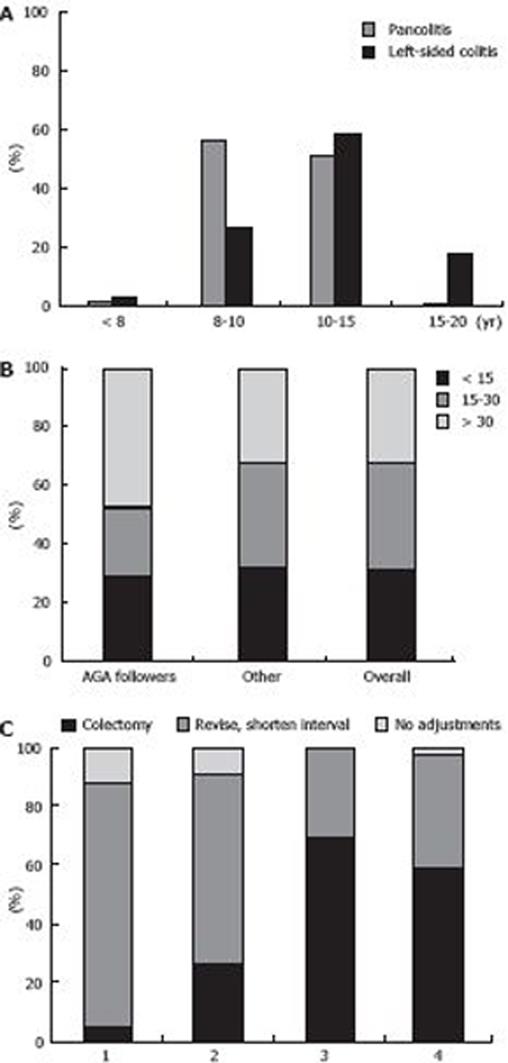
When asked how the duration of disease influenced their policy on commencement of surveillance for pancolitis or left-sided colitis, 53% of the GEs stated that they initiated colonoscopic screening for pancolitis after 8 to 10 years while 44% started screening after 10 to 15 years for left-sided colitis, which is in line with the AGA guidelines (Figure 1A).
The extent of colitis also plays a role[39]. Six percent of the GEs would screen patients with disease activity limited to the rectum, 68% would screen patients with left-sided colitis, and 26% would screen only in the case of pancolitis.
When asked what other factors would influence their screening protocol, 42% of the GEs mentioned PSC, 30% mentioned a positive family history of CRC, co-morbidity and general health, while 28% of the GEs did not take any factor into consideration.
Fifty-three percent of the GEs performed colonoscopic surveillance every 1 to 2 years, which is consistent with the AGA guidelines, whilst 22% performed surveillance once every 3 years in the second decade, once every 2 years in the third decade, and once a year in the fourth decade which is consistent with the British guidelines, 14% performed surveillance once every 3 years, and the rest of the GEs performed surveillance at different intervals without a specific protocol.
Forty three percent of the respondents stated that they take biopsies every 10 cm in every quadrant. The remaining GEs took 2 to 4 biopsies from different bowel segments (cecum, colon ascendens, colon tranversum, colon descendens, sigmoid, and rectum). Only 27% of all the respondents obtained more than 30 biopsies per colonoscopy, as recommended by the guidelines and Rubin et al[10]. Overall, the mean number of biopsies taken was 24 (Figure 1B).
When asked which policy was adopted during the follow-up period when unifocal LGD, multifocal LGD, HGD, DALM or CRC was observed, the GEs responded as shown in Figure 1C. If dysplasia was confirmed, 47% of the GEs advised that they would revise the histopathology, while 40% advised that they would obtain new biopsies, and 13% advised that they would do both. In the case of a DALM, 60% of the GEs would take biopsies from the lesion and the surrounding area, 36% would take biopsies from the lesion only, and 4% would remove the lesion endoscopically.
If a subtotal colectomy was performed, 83% would screen the rectum, and 22% of the 83% would screen once every 4 to 5 years, 54% would screen once every 2 years, while 7% would screen, but not on a regular basis.
Most Dutch gastroenterologists perform endoscopic surveillance without following international recommended guidelines. Although, surveillance guidelines are widely used, there is no agreement on surveillance guidelines or a national surveillance protocol in Netherlands. We studied the surveillance practice of Dutch GEs using a postal questionnaire. Overall, 153 out of 244 questionnaires were returned, a response rate which was comparable to most questionnaire-based studies directed at physicians[11]. We, therefore, assume that this study gives a representative overview of the use, feasibility, and ability to follow surveillance guidelines in Netherlands and that the results reflect the practice of a representative number of Dutch GEs. It is possible that the GEs who answered this questionnaire were more in favour of surveillance than GEs that did not fill out the questionnaire, which might have led to information bias. All GEs who provided surveillance agreed that screening patients with UC is necessary. It appears from the literature that not only UC, but also Crohn’s colitis is associated with an increased risk of colorectal cancer and, therefore, most experts recommend the use of the same guidelines for both UC and Crohn’s colitis[46]. However, only 65% of Dutch GEs provide surveillance for patients with Crohn’s colitis.
We compared our results with the guidelines set by the AGA. Firstly, we observed a large discrepancy between answers from GEs regarding the principle of surveillance in general, and the responses they provided related to their exact employment of surveillance in daily practice. Furthermore, although both pancolitis and left-sided colitis are associated with an increased risk of CRC[3], a quarter of the GEs do not provide surveillance for patients with left-sided colitis. On the other hand, a small group of GEs considered disease activity limited to the rectum an indication for screening, although there are no data to support the concept that proctitis increases the risk of CRC. All these inconsistencies could result in inefficient surveillance and missed dysplasia or even cancer.
The time between onset of symptoms and confirmed diagnosis of IBD can also differ substantially. Although there is no consensus on this subject, this difference in opinion might potentially lead to a delay in screening of months or even years.
Another important aspect of surveillance for CRC in IBD is adherence to the biopsy protocol. The median number of biopsies taken amongst Dutch GEs was 24 (range 10-40), while only 27% of the GEs approached the recommended number of 33 random biopsies. This number of biopsies was estimated to be necessary to detect possible dysplasia with a sensitivity of 90%[10]. A similar questionnaire-based study in New Zealand showed a median number of 17 biopsies[12]. This again, will inevitably lead to a pronounced decrease in sensitivity, rendering the surveillance tool ineffective.
If dysplasia is detected histopathologically, there seems to be uncertainty as to how to proceed with clinical decision-making. In the case of unifocal LGD, most of the Dutch GEs would have the histopathology revised, and would shorten the time interval to the next colonoscopy. If multifocal LGD is detected, Dutch GEs hesitate to recommend immediate colectomy, but prefer to revise the histopathology by consulting another pathologist or order a new colonoscopy with biopsies. Another suggested option was to shorten the time interval between screenings. Although controversy exists regarding the treatment policy which should be adopted after diagnosing dysplasia in patients with colitis, most experts agree that in all cases of confirmed dysplasia a colectomy should be recommended. Even the presence of LGD, which is associated with CRC in 21.4%-54%, can be considered an indication for surgery[46]. There is a disconcertingly low referral rate for colectomy amongst Dutch GEs, and even more so when findings are compared with 3 similar questionnaire-based studies in New Zealand, United Kingdom and Canada[12–14]. It is remarkable that the referral rate is higher for LGD and much lower for HGD and DALM compared with the other studies. The difficulty in confirming dysplasia, the lack of consensus for management of LGD, and the underestimation of the potential risk of LGD and HGD may contribute to the cautious management of LGD and HDG in Dutch GEs. Another reason could be that in the UK and the USA, the guidelines have already been implemented, which would explain the higher referral rate of cases with HGD in these countries.
In conclusion, 95% of Dutch GEs offer some form of surveillance, but most do not adhere to international guidelines. This leads to a decreased sensitivity for dysplasia, rendering this surveillance practice less effective. Furthermore, the management of dysplasia, even in cases of DALM, is inconsistent and will potentially lead to delays in the diagnosis of carcinomas. We suspect that this deviation from the guidelines is a general phenomenon in clinical practice, and is not only restricted to the Netherlands. Implementation of national guidelines and education of GEs concerning all aspects of colonoscopic surveillance is of great importance and will lead to a more consistent and efficient surveillance practice.
Patients with longstanding ulcerative colitis (UC) and Crohn’s colitis harbour an increased risk of developing colorectal cancer. It is generally agreed that screening and surveillance is a rational strategy in these patients, although the optimal screening strategy and approach to managing outcomes is still being debated.
Data from recent studies and new endoscopic techniques have changed the concepts on which surveillance guidelines have been built. Still, surveillance will depend on colonoscopy and requires commitment from both patients and gastroenterologists (GEs). Implementation of widely accepted guidelines is indispensable in realising optimal efficacy of a surveillance protocol. The challenge is to acquire nationwide support; only this will lead to a more consistent and efficient surveillance practice.
The authors report that most Dutch GEs offer some form of surveillance, although the majority do not follow international guidelines. This potentially results in the delayed diagnosis of advanced neoplasia in these patients, such as Dysplasia Associated Lesion or Mass (DALM), high-grade dysplasia (HGD) and colorectal carcinoma (CRC). The data are in line with studies from the UK, Canada and New Zealand, and call for more awareness on the level of national gastroenterology associations and GEs alike.
Implementation of national guidelines and education of GEs concerning all aspects of colonoscopic surveillance is of great importance and will probably lead to a more consistent and efficient surveillance practice.
In this manuscript, authors describe the results of their questionnaire regarding the association between the risk of CRC in patients with inflammatory bowel disease (IBD) and endoscopic screening. They conclude that implementation of national guidelines and education of GEs concerning all aspects of colonoscopic surveillance in IBD patients is of great importance. The findings are of interest.
| 1. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [Cited in This Article: ] |
| 2. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357-359. [Cited in This Article: ] |
| 3. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [Cited in This Article: ] |
| 4. | Eaden JA, Mayberry JF. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut. 2002;51 Suppl 5:V10-V12. [Cited in This Article: ] |
| 5. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371-1385. [Cited in This Article: ] |
| 6. | Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544-560. [Cited in This Article: ] |
| 7. | Eaden J, Abrams K, McKay H, Denley H, Mayberry J. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol. 2001;194:152-157. [Cited in This Article: ] |
| 8. | Melville DM, Jass JR, Morson BC, Pollock DJ, Richman PI, Shepherd NA, Ritchie JK, Love SB, Lennard-Jones JE. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum Pathol. 1989;20:1008-1014. [Cited in This Article: ] |
| 9. | Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590-1592. [Cited in This Article: ] |
| 10. | Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611-1620. [Cited in This Article: ] |
| 11. | Cummings SM, Savitz LA, Konrad TR. Reported response rates to mailed physician questionnaires. Health Serv Res. 2001;35:1347-1355. [Cited in This Article: ] |
| 12. | Gearry RB, Wakeman CJ, Barclay ML, Chapman BA, Collett JA, Burt MJ, Frizelle FA. Surveillance for dysplasia in patients with inflammatory bowel disease: a national survey of colonoscopic practice in New Zealand. Dis Colon Rectum. 2004;47:314-322. [Cited in This Article: ] |
| 13. | Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc. 2000;51:123-128. [Cited in This Article: ] |
| 14. | Bernstein CN, Weinstein WM, Levine DS, Shanahan F. Physicians’ perceptions of dysplasia and approaches to surveillance colonoscopy in ulcerative colitis. Am J Gastroenterol. 1995;90:2106-2114. [Cited in This Article: ] |