Published online Nov 28, 2009. doi: 10.3748/wjg.15.5549
Revised: September 12, 2009
Accepted: September 19, 2009
Published online: November 28, 2009
AIM: To investigate the effect of short-chain fatty acids (SCFAs) on production of prostaglandin E2 (PGE2), cytokines and chemokines in human monocytes.
METHODS: Human neutrophils and monocytes were isolated from human whole blood by using 1-Step Polymorph and RosetteSep Human Monocyte Enrichment Cocktail, respectively. Human GPR41 and GPR43 mRNA expression was examined by quantitative real-time polymerase chain reaction. The calcium flux assay was used to examine the biological activities of SCFAs in human neutrophils and monocytes. The effect of SCFAs on human monocytes and peripheral blood mononuclear cells (PBMC) was studied by measuring PGE2, cytokines and chemokines in the supernatant. The effect of SCFAs in vivo was examined by intraplantar injection into rat paws.
RESULTS: Human GPR43 is highly expressed in human neutrophils and monocytes. SCFAs induce robust calcium flux in human neutrophils, but not in human monocytes. In this study, we show that SCFAs can induce human monocyte release of PGE2 and that this effect can be enhanced in the presence of lipopolysaccharide (LPS). In addition, we demonstrate that PGE2 production induced by SCFA was inhibited by pertussis toxin, suggesting the involvement of a receptor-mediated mechanism. Furthermore, SCFAs can specifically inhibit constitutive monocyte chemotactic protein-1 (MCP-1) production and LPS-induced interleukin-10 (IL-10) production in human monocytes without affecting the secretion of other cytokines and chemokines examined. Similar activities were observed in human PBMC for the release of PGE2, MCP-1 and IL-10 after SCFA treatment. In addition, SCFAs inhibit LPS-induced production of tumor necrosis factor-α and interferon-γ in human PBMC. Finally, we show that SCFAs and LPS can induce PGE2 production in vivo by intraplantar injection into rat paws (P < 0.01).
CONCLUSION: SCFAs can have distinct antiinflammatory activities due to their regulation of PGE2, cytokine and chemokine release from human immune cells.
- Citation: Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou CC, Lundell D, Jenh CH. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E2 and cytokines. World J Gastroenterol 2009; 15(44): 5549-5557
- URL: https://www.wjgnet.com/1007-9327/full/v15/i44/5549.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5549
The receptors for free fatty acids, including GPR40, GPR41, GPR42 and GPR43, constitute a subfamily of recently deorphanized G-protein-coupled receptors (GPCRs) that are clustered on human chromosome 19q13.1[1]. They exhibit 30%-40% homology to one another and have diverse tissue distributions, yet all are activated by various free fatty acids, which function as intercellular lipid mediators through GPCRs[2,3].
The free fatty acid receptors are functionally involved in both metabolism and the immune system. In contrast to GPR40 which is activated by medium- and long-chain fatty acids and abundantly expressed in the pancreatic islets[4-7], GPR41 and GPR43 are functionally activated by short-chain fatty acids (SCFAs), including acetate, propionate and butyrate[8-10]. Although both receptors are activated by SCFAs, they have distinct tissue distribution profiles. GPR41 is preferentially expressed in adipose tissue, while GPR43 is highly expressed in hematopoietic tissues (such as spleen and bone marrow) and immune cells (particularly monocytes and neutrophils). Interestingly, the murine ortholog of GPR43 was initially identified as leukocyte-specific STAT-induced GPCR (LSSIG). It was reported that expression of both murine LSSIG and human GPR43 is induced during the differentiation of leukocyte progenitor cells to monocytes or neutrophils, suggesting their involvement in leukocyte function and host defense[11].
SCFAs have long been known to modulate the immune response. Acetate, propionate and butyrate represent the most often described SCFAs that are capable of immune activation. SCFAs affect neutrophil function and migration[12-15], and inhibit tumor necrosis factor-α (TNF-α) or interleukin-1β (IL-1β)-induced vascular cell adhesion molecule-1 (and intercellular adhesion molecule-1) surface expression[16]. In addition, SCFAs reduce adherence of monocytes or lymphocytes to cytokine-stimulated human umbilical vein endothelial cells[17], and inhibit interferon-γ (IFN-γ) signaling[18,19], a possible regulator of inflammation in inflammatory bowel disease (IBD). Although the molecular mechanisms of the effects of SCFAs remain to be elucidated, evidence in the literature indicates that SCFA-induced biological effects involve G-protein-mediated signaling[12,20]. GPR43, which is highly expressed in immune cells, could be the potential missing GPCR activated by SCFAs. GPR41 is also expressed in immune cells, but at lower levels compared to GPR43 and thus may play a lesser role[8-10]. As there are no well-validated specific drugs that can be used as tools to investigate the receptor, the emerging biology of these receptors is dependent on expression profiling. It is believed that GPR43 is likely the main receptor contributing to the effect of SCFAs in immune cells. In human neutrophils, it was reported that SCFAs induced calcium flux and chemotaxis possibly through GPR43[9]. Nonetheless, the precise role of GPR43 in the effect of SCFAs in neutrophils needs to be established with the generation of a knockout model and specific small molecules. In addition, GPR43 is also highly expressed in monocytes and peripheral blood mononuclear cells (PBMC)[9], and the effects of SCFAs in these important immune cells are of high interest, but are largely unknown.
SCFAs are produced by microbial anaerobic fermentation in the hindgut in millimolar (mmol/L) concentrations; therefore, the physiological site of GPR43 activation may be in the gut. Within the human colon, concentrations of acetate, propionate and butyrate were reported to be in the range of 20-43 mmol/L, 6-13 mmol/L and 6-15 mmol/L, respectively[21]. GPR43 has been reported to be expressed in human, mouse and rat colons[22-24]. Induction of antiinflammatory activities by SCFAs in the colon may contribute to the bacterial evasion of the immune system[25]. GPR43-mediated activation may provide a link between the intestinal bacteria and the immune response in physiological and pathophysiological conditions.
We report the identification of new functional roles of SCFAs in production of prostaglandin E2 (PGE2), cytokines and chemokines in human monocytes and in PBMC. We speculate that the effect of SCFAs is most likely through activation of GPR43, a potential therapeutic target for the development of an innovative treatment for colitis.
Human neutrophils were isolated from human whole blood by using 1-Step Polymorph (Accurate Chemical & Scientific Corp., Westbury, NY) according to the manufacturer’s instructions. Human monocytes were isolated by RosetteSep Human Monocyte Enrichment Cocktail (StemCell Technologies, London, UK). Human PBMC were prepared by the Ficoll-Hypaque centrifugation method.
For the calcium flux assay, both human neutrophils and monocytes were treated with formate, acetate and propionate (Sigma, St. Louis, MO, USA) for 5-10 min. For measurement of PGE2, cytokines and chemokines, human monocytes and PBMC cells were incubated with formate, acetate, propionate and butyrate (Sigma, St. Louis, MO, USA) overnight.
TaqMan primers and probes were designed with Primer Express software and purchased from ABI (Applied Biosystems, Foster City, CA, USA). The sequences of the human primers and probes for GPR41 and GPR43 are shown in Table 1. Quantitative real-time PCR was carried out with an ABI Prism 7900HT Sequence Detection System. The PCR reactions were prepared using the components from the iScript Custom One-Step RT-PCR Kit with ROX and assembled according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). The final concentrations of the primers and probe in the PCR reactions were 200 nmol/L and 100 nmol/L, respectively. The fluorogenic probes were labeled with 6-carboxyfluorescein as the reporter and 6-carboxy-4,7,2,7’-tetramethylrhodamine as a quencher. Each 10 μL PCR reaction contained 10 ng of total RNA. The RT-PCR reactions were performed in triplicate in a 384-well plate according to the following protocol: one cycle for 10 min at 50°C, followed by one 5 min cycle at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. A eukaryotic 18S rRNA endogenous control probe/primer set (ABI) was used as an internal control for RNA quality.
| Gene | RefSeq ID | Forward primer | Reverse primer | Probe |
| GPR41 | NM_005304 | GCTTTGGGCCCTACAACGT | CCATGCCGGGCTTTCA | TCCCATGTCGTGGGCTATATCTGCG |
| GPR43 | NM_005306 | GGCTTTCCCCGTGCAGTAC | ACCAGAGCTGCAATCACTCCAT | AGCTCTCCCGCCGGCCTCTG |
The PCR data were quantified, based on a 12-point standard curve generated using 4-fold serial dilutions of a cDNA containing the gene of interest. The 4-fold dilutions began at 20 000 fg. This procedure provides an absolute quantification of the amounts of GPR41 and GPR43 mRNA in a given sample.
Intracellular Ca2+ assays were carried out as previously described with modifications[26]. Freshly isolated human neutrophils were resuspended at 1 × 106 cells/mL in cell culture medium (RPMI 1640 with 2 mmol/L GlutaMAX and 5% fetal bovine serum) and incubated at 37°C, 50 mL/L CO2, for 1 h. Cells were spun and resuspended at 1 × 106 cells/mL in a 1:1 mixture of cell culture medium and Calcium-4 no wash dye (Molecular Devices, Sunnyvale, CA, USA) containing a final concentration of 5 mmol/L probenecid (Sigma). The cells were seeded (50 000/well) into poly-D-lysine-coated 384-well, black-wall, clear bottom microtiter plates (Becton Dickinson). Cells were sedimented in plates by spinning down briefly at low speed with no brake then incubated at 37°C, 50 mL/L CO2, for 1 h before assaying on a FLIPR-384 (Molecular Devices). Freshly isolated human monocytes were resuspended at 1 × 109 cells/L in a 1:1 mixture of cell culture medium and Calcium-3 no wash dye containing a final concentration of 2.5 mmol/L probenecid. The cells were seeded (50 000/well) into poly-D-lysine coated 384-well plates. Cells were dye loaded for 45 min at 37°C, 50 mL/L CO2, before assaying on a FLIPR-384.
Formate, acetate, propionate and butyrate (Sigma) were prepared in Hank’s balanced salt solution, 25 mmol/L Hepes, and 0.1% bovine serum albumin. The maximum change in fluorescence over baseline was used to determine agonist response. The dose-response curves and EC50 values were obtained by nonlinear regression (Prism 4; Graph Pad Software, San Diego, CA, USA).
PGE2 was measured by ELISA kit from Assay Designs (Ann Arbor, MI, USA). Cytokines and chemokines were measured by MesoScale ELISA (MesoScale Discovery, Gaithersburg, MD, USA) according to the manufacturer’s instructions. To generate the conditioned media, 1 × 106 cells per mL were resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1 mmol/L L-glutamine, 10 mmol/L Hepes, 50 000 U/L penicillin and 50 μg/mL streptomycin.; and seeded in 24-well plates for stimulation with SCFAs and/or incubation with selective inhibitors (Cayman Chemical, Ann Arbor, MI, USA), including MLnFP (methyl α-linolenyl fluorophosphonate), a phospholipase inhibitor; 1400W, a selective iNOS inhibitor; SC-560, a selective cyclooxygenase-1 (COX-1) inhibitor; CAY10404, a selective COX-2 inhibitor; PTX, pertussis toxin. The tested concentrations of each inhibitor (except PTX) was chosen to be about 100 times of its IC50 as reported in the data sheet provided by the manufacturer.
Sprague-Dawley male rats from Charles River Laboratories, 4 per group and approximately 200 g body weight, were anesthetized with isoflurane gas and the subplantar space of the paw injected with 0.1 mL of 100 mmol/L solutions of the SCFAs, either formate, acetate, propionate or butyrate prepared fresh in 0.9% saline. Lipopolysaccharide (LPS), Escherichia coli 0127:B8 (Sigma) was also injected at 3 μg in saline either alone or in combination with 0.1 mL of 200 mmol/L sodium butyrate. Rats in the normal group were not injected. At 3 h post-injection, the rats were humanely euthanized and a uniform punch biopsy of the injected site was taken from each rat. The punch biopsies were immediately placed in PMSF (phenylmethanesulphonyl fluoride) buffer containing 10 g/L of indomethacin and frozen at -20°C. The tissues were homogenized in this collection buffer and assayed for PGE2.
All statistical analysis was performed by Mann-Whitney U test using GraphPad Instat version 3.06 for Windows XP (GraphPad Software, San Diego, CA, USA). All studies in animals were performed in accordance with the regulations specified by the National Institutes of Health Principles of Laboratory Animal Care (1985 revised version) and the Schering-Plough Research Institute Animal Care and Use Committee.
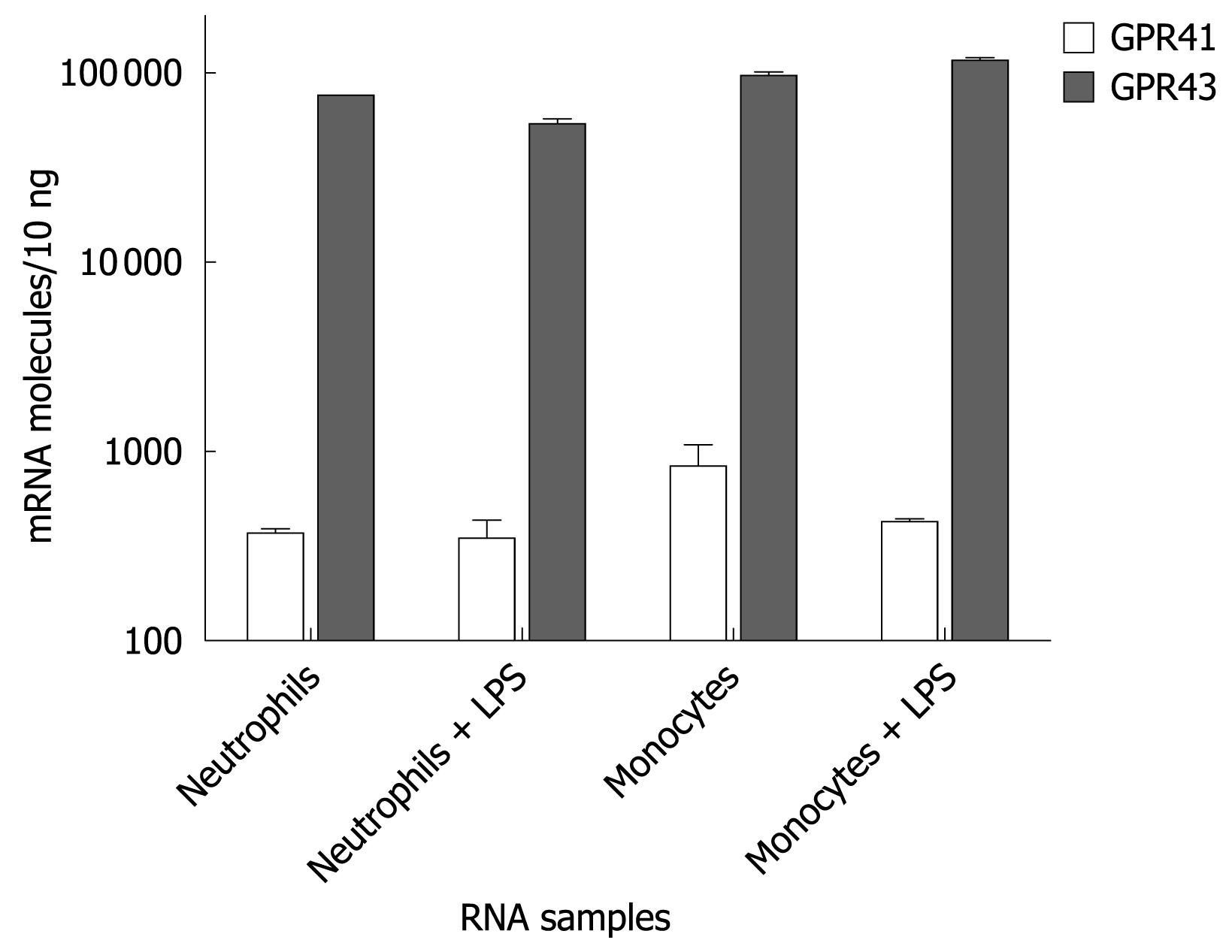
Both GPR43 and GPR41 are activated by SCFAs and reported to be expressed in immune cells. To examine the role of GPR43 and GPR41 in human immune cells, we initially quantified their expression levels in human neutrophils and monocytes by Taqman analysis. Human neutrophils and monocytes were each isolated from human donors to 95% purity. Some of them were stimulated with LPS. RNAs were isolated and analyzed for GPR43 and GPR41 expression by Taqman. Figure 1 shows that GPR43 is expressed in both human neutrophils and monocytes at a much higher level than GPR41. It also appears that LPS stimulation did not affect their expression levels.
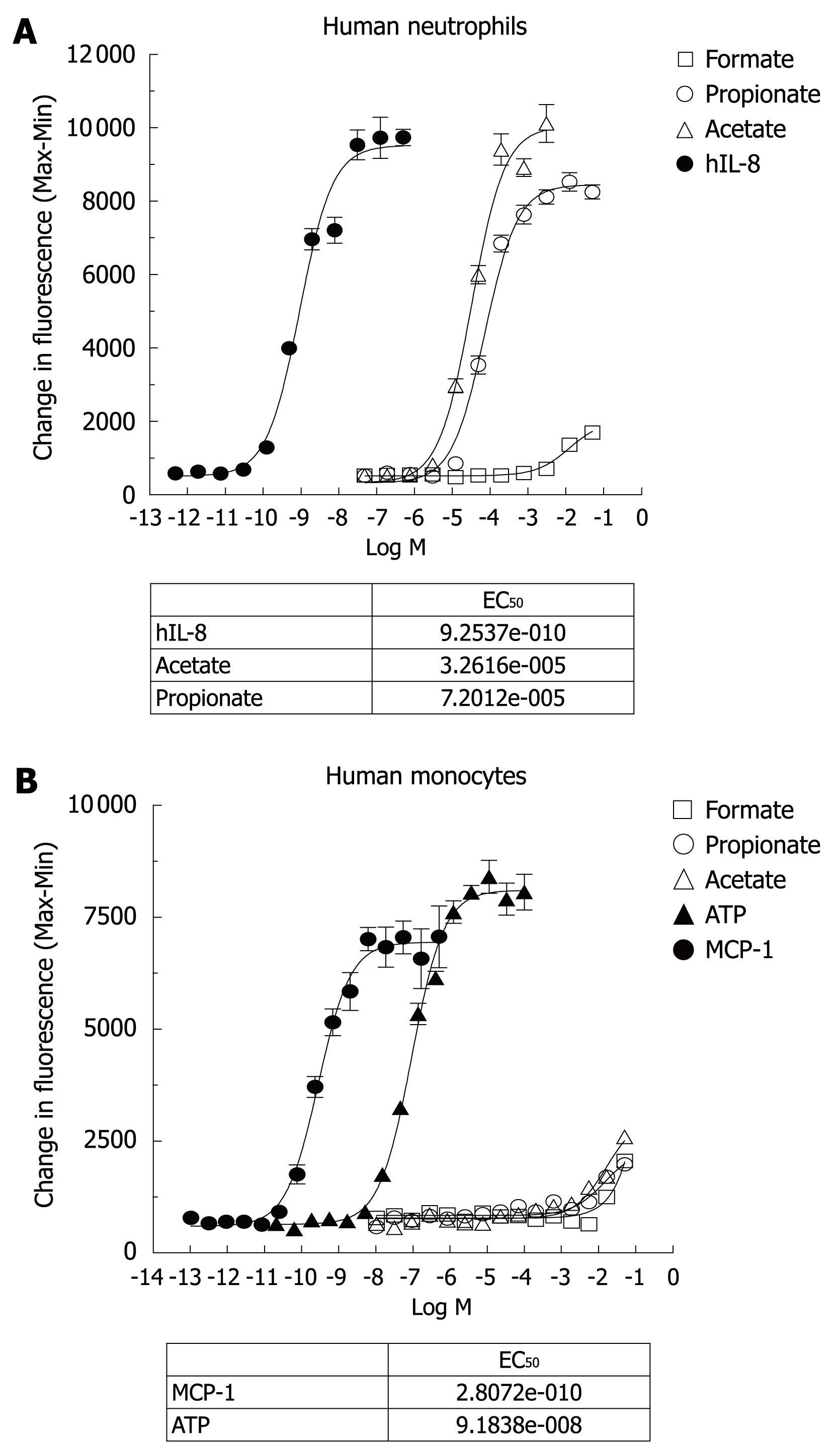
To investigate the biological activities of SCFAs, both purified human neutrophils and monocytes were exposed to various concentrations of SCFAs (formate, acetate and propionate) in a calcium flux assay. Formate was used as a negative control for the SCFAs. In addition, IL-8 was included as a positive control for neutrophil activation, while monocyte chemotactic protein-1 (MCP-1) and ATP were used as the positive controls for monocyte activation. Since GPR41 couples to Gi/o only, SCFAs should not cause a calcium flux through this receptor, which was confirmed in a recombinant cell line expressing GPR41 (data not shown). Indeed, the agonist potency profile of the calcium response in human neutrophils (Figure 2A) was consistent with the GPR43 receptor response that has been described[9]. From 8 human donors, acetate had an average EC50 of 58.25 ± 12.44 μmol/L (n = 8), and propionate had an EC50 of 200.6 ± 42.42 μmol/L (n = 8). Formate was inactive and IL-8 had an EC50 about 1-2 nmol/L in human neutrophils. Acetate and propionate achieved the same maximal activation response as IL-8 in human neutrophils using this calcium flux assay.
On the other hand, Figure 2B shows that acetate and propionate, up to 100 mmol/L, did not induce significant calcium flux in human monocytes, while MCP-1 and ATP were able to induce robust calcium flux in the human monocyte preparation. It appears that the response of GPR43 to SCFAs for calcium flux is dependent either on cell type or on the state of differentiation of the cells. Indeed, it has been reported that propionic acid induced calcium mobilization in human neutrophils, but not in monocytes, platelets and lymphocytes[12].
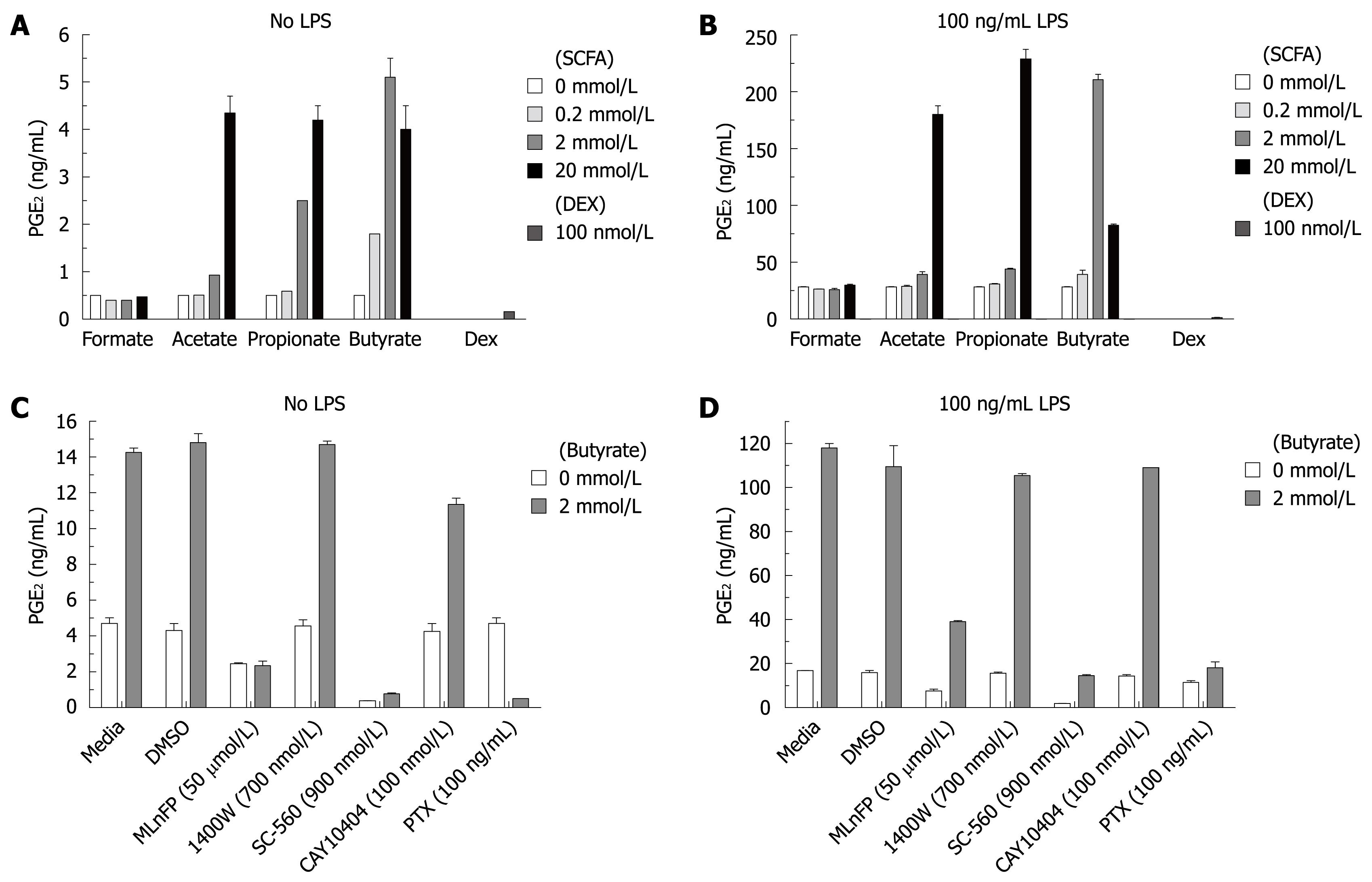
To further explore the biological effects of SCFAs, human monocytes were isolated and incubated overnight with various concentrations of SCFAs in the presence or absence of LPS (100 ng/mL) as a proinflammatory stimulus. The supernatants were assayed for lipid mediators such as PGE2, proinflammatory cytokines and chemokines by either ELISA or Meso Scale Multi-Spot Discovery Technology. Dexamethasone was included in the experiment as a positive control for the induction of inhibition. Figure 3A shows that SCFAs alone strongly enhanced PGE2 production in human monocytes across a panel of 10 human donors in a concentration-dependent manner. Furthermore, SCFAs synergistically enhanced the PGE2 production induced by LPS in human monocytes (10 human donors), as shown in Figure 3B. The rank order of potency was butyrate > propionate > acetate, while formate was inactive. Dexamethasone at 100 nmol/L potently inhibited basal PGE2 and LPS-induced PGE2 production (Figure 3A and B). Similar results were obtained from 10 human donors. The culture supernatants were also assayed for PGI2, leukotriene B4 (LTB4) and thromboxane B2 (TXB2). SCFAs had no significant effect on these lipid mediators (data not shown), suggesting its effect on PGE2 production is specific.
To examine possible mechanisms of PGE2 production induced by SCFAs, human monocytes were preincubated with several inhibitors and then added to culture with butyrate and/or LPS. Figure 3C and D show that the PGE2 production induced by butyrate is inhibited by pretreatment with PTX, COX-1 and phospholipase inhibitors either in the absence or presence of LPS. In contrast, COX-2 and iNOS inhibitors had no effect on PGE2 production induced by butyrate. The sensitivity to PTX suggests PGE2 production induced by butyrate is receptor-mediated.
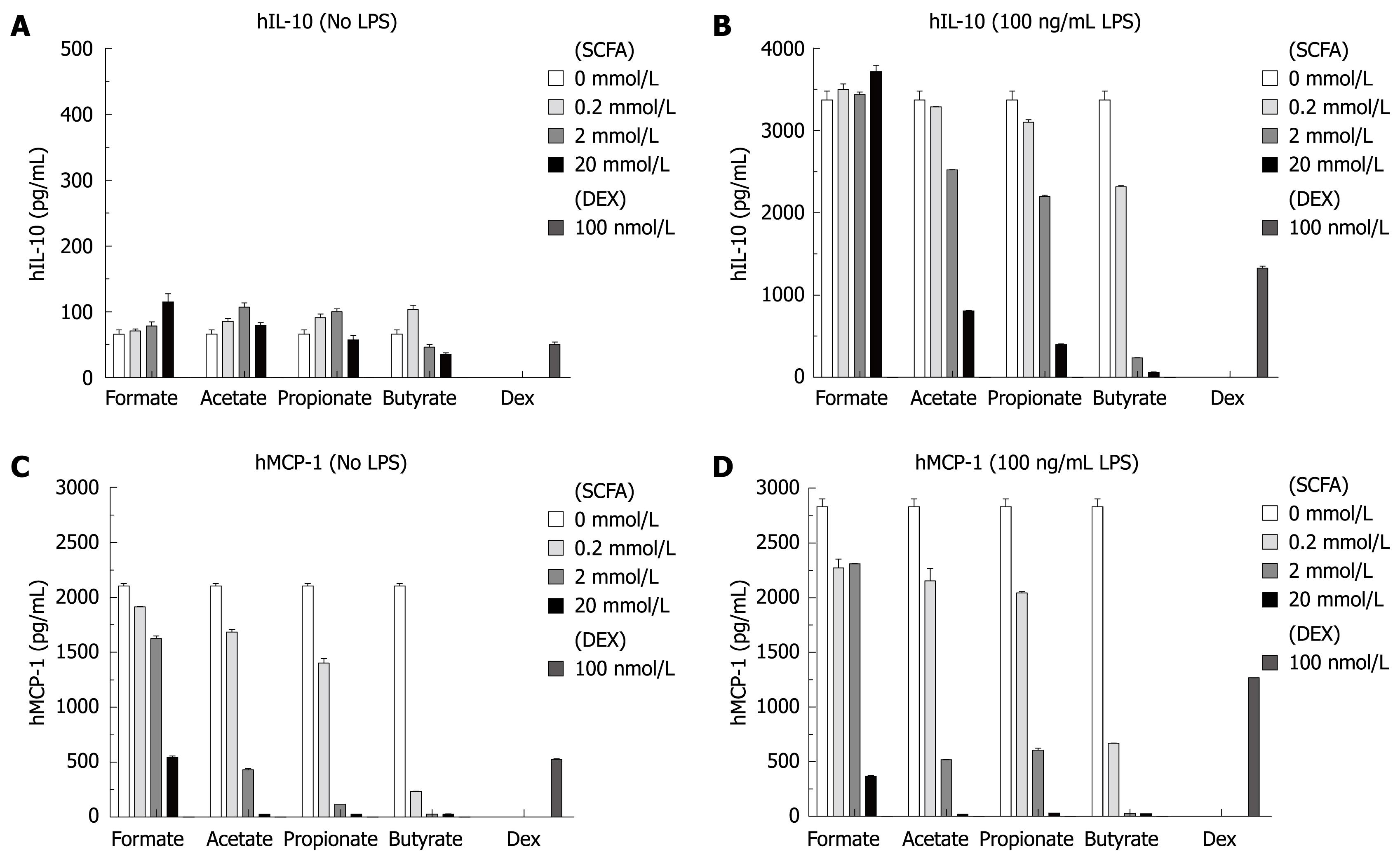
The same culture supernatants described above were also analyzed for 8 proinflammatory cytokines including IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IFN-γ, TNF-α (Meso Scale Multi-Spot Discovery Technology). In the absence of LPS, SCFAs had no effect on the level of any of the 8 cytokines including IL-10, as shown in Figure 4A. In contrast, SCFAs specifically inhibited IL-10 production stimulated with LPS (Figure 4B), but had no effect on the other 7 cytokines with LPS stimulation (including LPS-induced production of IL-1β and TNF-α. The effect of dexamethasone on the cytokine profile is consistent with what has been previously described; briefly, it inhibited LPS-induced production of IL-1β, IL-10 and TNF-α.
It is consistently observed that isolated human monocytes produce high levels of MCP-1 in the culture supernatants without stimulation. Figure 4C and D show that SCFAs inhibited this constitutive MCP-1 production in a concentration-dependent manner either in the absence or presence of LPS. SCFAs did not inhibit the constitutive high levels of eotaxin-3, macrophage-derived chemokine and macrophage inflammatory protein-3β in the cultures, suggesting that the effect of SCFAs on MCP-1 production is specific. In this experiment, 20 mmol/L formate showed some inhibition, which is likely the result of a nonspecific effect.
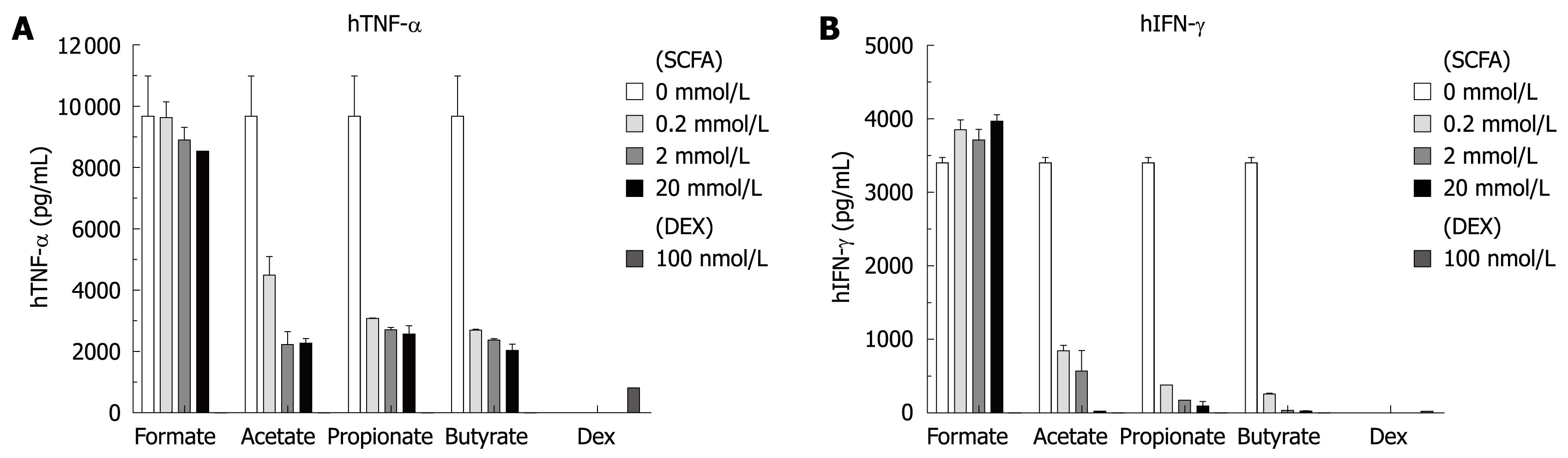
To further confirm the effect of SCFAs, human PBMC were isolated by Ficoll-Hypaque centrifugation procedure and incubated with SCFAs and/or LPS. The culture supernatants were analyzed as before for PGE2, cytokines and chemokines. The effects of SCFAs on PGE2, MCP-1 and IL-10 production were similar to that observed in the isolated human monocytes. However, as shown in Figure 5, SCFAs inhibited LPS-induced production of TNF-α and IFN-γ in human PBMC in a concentration-dependent manner, an effect not observed in isolated human monocytes where IFN-γ production was low and not stimulated by LPS addition. In the human PBMC experiment, dexamethasone inhibited LPS-induced production of IL-1β, IL-10, IFN-γ and TNF-α.
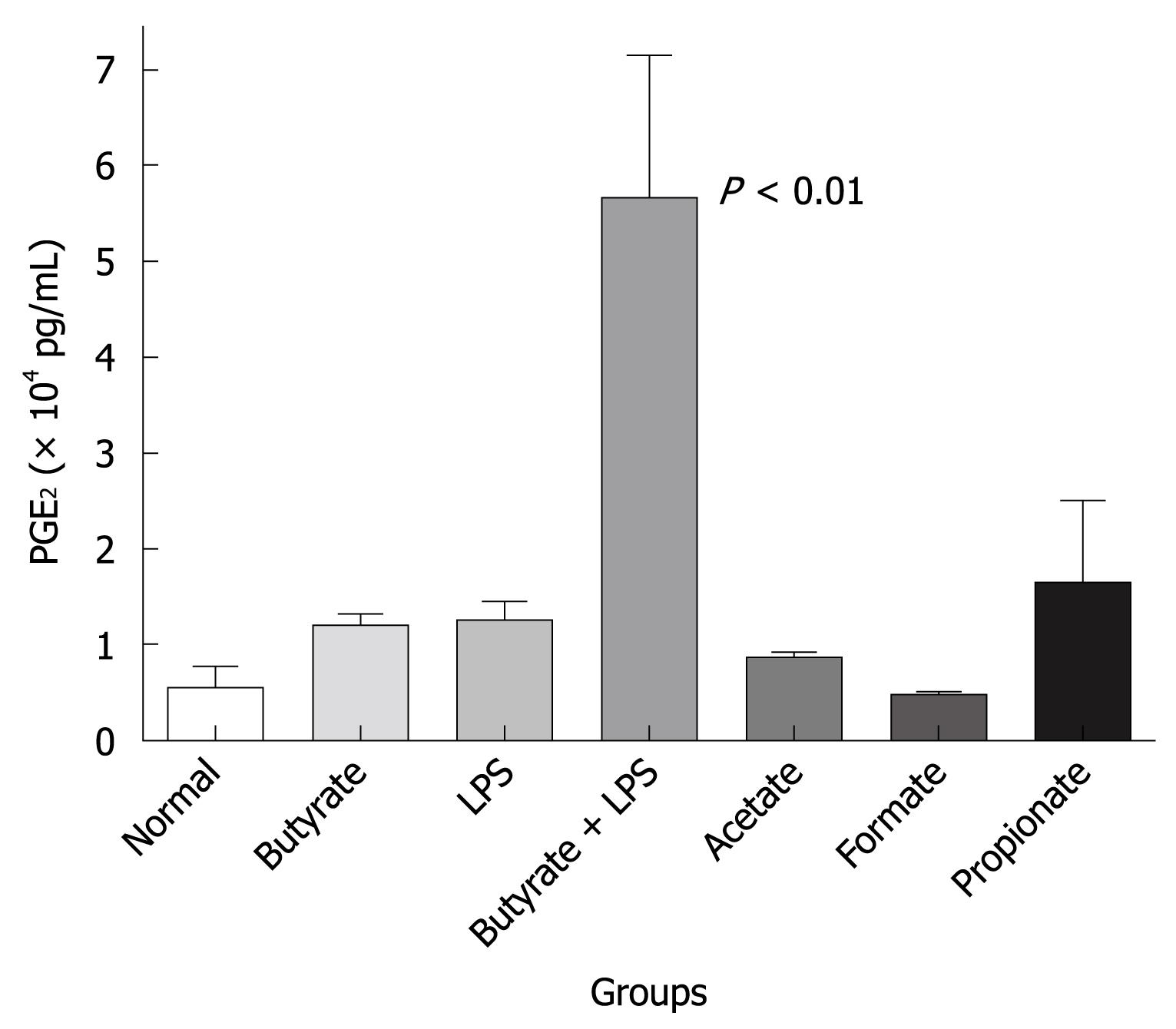
To demonstrate the effect of SCFAs in vivo, SCFAs and LPS alone or in combination were injected by the intraplantar route into a rat paw. This was done to initiate an inflammatory response and determine what effect the butyrate would have on lipid mediator and cytokine induction. In the representative experiment shown in Figure 6, acetate, propionate and butyrate alone could induce PGE2 production in rat paw. Formate was inactive and the level of PGE2 was equivalent to that extracted from a normal (not injected) paw. In addition, suboptimal LPS also induced PGE2 production and the combination of LPS and butyrate strongly enhanced PGE2 production in a synergistic manner (P < 0.01). These in vivo data, although preliminary in nature, support the effects of SCFAs on PGE2 production observed in human monocytes and PBMC. Cytokine analysis was performed on the tissue homogenates but no clear pattern emerged that was similar to that obtained in the human monocytes and PBMC in vitro experiments. Sodium butyrate is extremely labile with a very high diffusion rate. Maintenance of a sufficient concentration in the paw subcutaneous space was a significant problem in these experiments.
In this study, we have shown that SCFAs can induce either robust PGE2 production alone or in synergy with LPS in human monocytes and PBMC. Since LPS stimulation did not affect the mRNA level of GPR43 and GPR41, the synergy is likely from 2 independent signaling pathways. This effect on PGE2 production is specific, since other lipid mediators including PGI2, LTB4 and TXB2, were not affected by SCFAs. Furthermore, this effect is receptor-mediated since it is sensitive to PTX.
Interestingly, we showed that SCFAs induced robust calcium flux in human neutrophils, but not in human monocytes. Our finding is consistent with the previous report that propionic acid induced calcium mobilization in human neutrophils, but not in monocytes, platelets and lymphocytes[12]. It is likely that SCFAs activate neutrophils and monocytes for the immune response through different signaling pathways that would be consistent with the different temporal roles of these cells in an inflammatory response. SCFAs induce calcium flux and chemotaxis in neutrophils which arrive early in an inflammatory response, while they regulate production of PGE2, cytokines and chemokines in monocytes which are also present in the inflammatory site but mature into macrophages during extravasation and remain at the site of inflammation until resolution.
This in vitro effect of SCFAs on human monocytes may have relevance to human diseases that target the intestinal tract. Among various prostanoids, PGE2, in particular, seems to play a critical role in inflammatory bowel disease (IBD) via the EP4 receptor, one of the four PGE2 receptor subtypes(EP1-4)[27-31]. Among 8 prostanoid receptor-deficient mice, only EP4-deficient mice developed severe colitis with 3% dextran sodium sulphate (DSS) treatment. It is suggested that EP4 maintains intestinal homeostasis by maintaining mucosal integrity and downregulating the immune response[32]. Therefore, it is possible that SCFAs induce production of PGE2 and play a protective role via the GPR43-expressing cells of the colon. Furthermore, we demonstrate that PGE2 production induced by SCFAs was inhibited by a COX-1 inhibitor, but not a COX-2 inhibitor. This is consistent with the notion that in DSS-induced colitis, the significant reduction in PGE2 resulted from decreased expression of COX-1, but not COX-2[33].
In human monocytes, SCFAs specifically inhibited constitutive MCP-1 production and LPS-induced IL-10 production out of the total 16 cytokines and chemokines examined. The activities of SCFAs in our human monocyte cultures were all confirmed in human PBMC, which contain both monocytes and lymphocytes. We made the additional observation in these PBMC cultures that SCFAs would inhibit LPS-induced TNF-α and IFN-γ production.
This unique effect of SCFAs on the production of cytokines and chemokines in monocytes and PBMC is relevant to the design of new therapies for colitis. Intestinal inflammation present in inflammatory bowel disease is driven by the production of cytokines, chemokines and growth factors that draw in immune cells to the mucosa. It has been shown that MCP-1, IL-10, IFN-γ and TNF-α were significantly upregulated in experimental colitis models[34,35]. Furthermore, the increased production of SCFAs from dietary fiber supplementation or probiotics administration inhibited the production of proinflammatory mediators and recovered damaged colonic mucosa in colitic animals[36,37]. These experiments show that SCFAs can inhibit multiple inflammatory mediators which may control intestinal inflammation. Clinical trial evidence for this use of SCFAs is still not widely available or accepted as a mainstream therapy.
We tried to design a model system in vivo to duplicate our in vitro findings. We present the results of a model system using the intraplantar space in the rat paw, a space from which there was limited diffusion of the injected SCFAs. We had previously tried injecting SCFAs into the pleural cavity and into the peritoneal cavity to induce an inflammatory response with and without suboptimal inflammatory stimuli such as LPS. However, SCFAs are extremely labile with a very high diffusion rate and require high concentrations to achieve their effect in vivo. Maintenance of a sufficient concentration of SCFAs at a local site or tissue space (such as the colon) has been a major challenge to obtain clear cytokine profiles and in vivo efficacy. Therefore, better pharmacological tools, such as GPR43 specific small molecules[38], are needed to confirm our in vitro findings and further elucidate their biology. We were able to utilize the intraplantar space of the paw to illustrate the ability of SCFAs to synergize with LPS and induce mediator production but there was significant variability in this response.
The present findings of antiinflammatory activities of SCFAs in immune cells expand the database on these fatty acids as lipid mediators and may help explain the known beneficial effects of SCFAs in the colon and on colitis. Future studies are needed in knockout mice and with specific small molecules (both agonists and antagonists) to prove the exact molecular target for these effects of SCFAs.
Short-chain fatty acids (SCFAs) are produced by bacterial fermentation of dietary fiber in the hindgut in millimolar concentrations. They have long been known to modulate the immune response and have a beneficial effect in the colon and on colitis.
The molecular mechanism for the effect of SCFAs in immune cells of the gastrointestinal tract has not been characterized. Recently, a free fatty acid receptor GPR43 was found to be highly expressed in both neutrophils and monocytes and identified to be activated by SCFAs. In neutrophils, SCFAs induce calcium flux and chemotaxis. However, the effect of SCFAs in monocytes is of high interest, but is largely unknown.
This study showed that SCFAs can induce specific and robust prostaglandin E2 (PGE2) production either alone or in synergy with lipopolysaccharide in human monocytes and peripheral blood mononuclear cells (PBMC). Furthermore, SCFAs can specifically regulate production of monocyte chemotactic protein-1, interleukin-10, tumor necrosis factor-α and interferon-γ in human monocytes and/or PBMC. Finally, we showed that SCFAs can induce PGE2 production in vivo by intraplantar injection into rat paws.
The present findings of antiinflammatory activities of SCFAs in immune cells expand the database on these fatty acids and may help explain the known beneficial effects of SCFAs in the colon and on colitis. Future studies are needed in knockout mice and with specific small molecules to prove the exact molecular target for these effects of SCFAs. The authors speculate that the action of SCFAs is most likely through activation of the free fatty acid receptor GPR43, a potential therapeutic target for the development of an innovative treatment of colitis.
This is a study in an important area of inflammatory activities of SCFAs and its effects on the related cytokines and chemokines. In the present paper, the authors examined the inflammatory activities of SCFAs and the production of the related receptors and analyzed the mechanisms. The experiments were well organized.
Peer reviewers: Wei Tang, MD, EngD, Assistant Professor, H-B-P Surgery Division, Artificial Organ and Transplantation Division, Department of surgery, Graduate School of Medicine, The University of Tokyo, Tokyo 113-8655, Japan; Dr. Dae-Yeul Yu, Professor, 111 Gwahangno, Yuseong-gu, 305-806 Daejeon, South Korea
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
| 1. | Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O'Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun. 1997;239:543-547. [Cited in This Article: ] |
| 2. | Im DS. Discovery of new G protein-coupled receptors for lipid mediators. J Lipid Res. 2004;45:410-418. [Cited in This Article: ] |
| 3. | Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA Cell Biol. 2005;24:54-61. [Cited in This Article: ] |
| 4. | Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303-11311. [Cited in This Article: ] |
| 5. | Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173-176. [Cited in This Article: ] |
| 6. | Kotarsky K, Nilsson NE, Olde B, Owman C. Progress in methodology. Improved reporter gene assays used to identify ligands acting on orphan seven-transmembrane receptors. Pharmacol Toxicol. 2003;93:249-258. [Cited in This Article: ] |
| 7. | Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245-258. [Cited in This Article: ] |
| 8. | Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312-11319. [Cited in This Article: ] |
| 9. | Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481-25489. [Cited in This Article: ] |
| 10. | Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047-1052. [Cited in This Article: ] |
| 11. | Senga T, Iwamoto S, Yoshida T, Yokota T, Adachi K, Azuma E, Hamaguchi M, Iwamoto T. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood. 2003;101:1185-1187. [Cited in This Article: ] |
| 12. | Naccache PH, Faucher N, Caon AC, McColl SR. Propionic acid-induced calcium mobilization in human neutrophils. J Cell Physiol. 1988;136:118-124. [Cited in This Article: ] |
| 13. | Nakao S, Fujii A, Niederman R. Alteration of cytoplasmic Ca2+ in resting and stimulated human neutrophils by short-chain carboxylic acids at neutral pH. Infect Immun. 1992;60:5307-5311. [Cited in This Article: ] |
| 14. | Nakao S, Moriya Y, Furuyama S, Niederman R, Sugiya H. Propionic acid stimulates superoxide generation in human neutrophils. Cell Biol Int. 1998;22:331-337. [Cited in This Article: ] |
| 15. | Böcker U, Nebe T, Herweck F, Holt L, Panja A, Jobin C, Rossol S, B Sartor R, Singer MV. Butyrate modulates intestinal epithelial cell-mediated neutrophil migration. Clin Exp Immunol. 2003;131:53-60. [Cited in This Article: ] |
| 16. | Zapolska-Downar D, Siennicka A, Kaczmarczyk M, Kołodziej B, Naruszewicz M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: the role of NF-kappaB and PPARalpha. J Nutr Biochem. 2004;15:220-228. [Cited in This Article: ] |
| 17. | Menzel T, Lührs H, Zirlik S, Schauber J, Kudlich T, Gerke T, Gostner A, Neumann M, Melcher R, Scheppach W. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm Bowel Dis. 2004;10:122-128. [Cited in This Article: ] |
| 18. | Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res. 2003;1:855-862. [Cited in This Article: ] |
| 19. | Park JS, Lee EJ, Lee JC, Kim WK, Kim HS. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int Immunopharmacol. 2007;7:70-77. [Cited in This Article: ] |
| 20. | Brunkhorst BA, Kraus E, Coppi M, Budnick M, Niederman R. Propionate induces polymorphonuclear leukocyte activation and inhibits formylmethionyl-leucyl-phenylalanine-stimulated activation. Infect Immun. 1992;60:2957-2968. [Cited in This Article: ] |
| 21. | Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031-1064. [Cited in This Article: ] |
| 22. | Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135-142. [Cited in This Article: ] |
| 23. | Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, Moore GB, Taylor CM, Sanger GJ. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19:66-74. [Cited in This Article: ] |
| 24. | Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353-360. [Cited in This Article: ] |
| 25. | Säemann MD, Böhmig GA, Zlabinger GJ. Short-chain fatty acids: bacterial mediators of a balanced host-microbial relationship in the human gut. Wien Klin Wochenschr. 2002;114:289-300. [Cited in This Article: ] |
| 26. | Cox MA, Gomes B, Palmer K, Du K, Wiekowski M, Wilburn B, Petro M, Chou CC, Desquitado C, Schwarz M. The pyrimidinergic P2Y6 receptor mediates a novel release of proinflammatory cytokines and chemokines in monocytic cells stimulated with UDP. Biochem Biophys Res Commun. 2005;330:467-473. [Cited in This Article: ] |
| 27. | Jiang GL, Nieves A, Im WB, Old DW, Dinh DT, Wheeler L. The prevention of colitis by E Prostanoid receptor 4 agonist through enhancement of epithelium survival and regeneration. J Pharmacol Exp Ther. 2007;320:22-28. [Cited in This Article: ] |
| 28. | Nitta M, Hirata I, Toshina K, Murano M, Maemura K, Hamamoto N, Sasaki S, Yamauchi H, Katsu K. Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: colitis suppression by a selective agonist, ONO-AE1-329. Scand J Immunol. 2002;56:66-75. [Cited in This Article: ] |
| 29. | Narumiya S. Prostanoids in immunity: roles revealed by mice deficient in their receptors. Life Sci. 2003;74:391-395. [Cited in This Article: ] |
| 30. | Matsuoka T, Narumiya S. Prostaglandin receptor signaling in disease. ScientificWorldJournal. 2007;7:1329-1347. [Cited in This Article: ] |
| 31. | Fujino H, Regan JW. EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol Pharmacol. 2006;69:5-10. [Cited in This Article: ] |
| 32. | Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, Tsuboi K, Sugimoto Y, Kobayashi T, Miyachi Y. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002;109:883-893. [Cited in This Article: ] |
| 33. | Martín AR, Villegas I, Alarcón de la Lastra C. The COX-2 inhibitor, rofecoxib, ameliorates dextran sulphate sodium induced colitis in mice. Inflamm Res. 2005;54:145-151. [Cited in This Article: ] |
| 34. | Sun FF, Lai PS, Yue G, Yin K, Nagele RG, Tong DM, Krzesicki RF, Chin JE, Wong PY. Pattern of cytokine and adhesion molecule mRNA in hapten-induced relapsing colon inflammation in the rat. Inflammation. 2001;25:33-45. [Cited in This Article: ] |
| 35. | Rodríguez-Cabezas ME, Gálvez J, Lorente MD, Concha A, Camuesco D, Azzouz S, Osuna A, Redondo L, Zarzuelo A. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J Nutr. 2002;132:3263-3271. [Cited in This Article: ] |
| 36. | Rodríguez-Cabezas ME, Gálvez J, Camuesco D, Lorente MD, Concha A, Martinez-Augustin O, Redondo L, Zarzuelo A. Intestinal anti-inflammatory activity of dietary fiber (Plantago ovata seeds) in HLA-B27 transgenic rats. Clin Nutr. 2003;22:463-471. [Cited in This Article: ] |
| 37. | Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailón E, Nieto A, Concha A, Olivares M, Zarzuelo A, Xaus J. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97:96-103. [Cited in This Article: ] |
| 38. | Lee T, Schwandner R, Swaminath G, Weiszmann J, Cardozo M, Greenberg J, Jaeckel P, Ge H, Wang Y, Jiao X. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol Pharmacol. 2008;74:1599-1609. [Cited in This Article: ] |