Published online Jan 7, 2012. doi: 10.3748/wjg.v18.i1.49
Revised: April 15, 2011
Accepted: April 22, 2011
Published online: January 7, 2012
AIM: To evaluate the prognosis of type II diabetes mellitus (T2DM) after gastrectomy and related factors in gastric cancer patients.
METHODS: 403 gastric cancer patients with T2DM were studied, who underwent gastrectomy between May 2003 and September 2009. A review of medical records and telephone interviews was performed in this cross-sectional study. The factors included in the statistical analysis were as follows: gender, age, type of surgery, preoperative body mass index (BMI), current BMI, BMI reduction ratio, preoperative insulin or oral diabetic medicine requirement, follow-up duration, and current state of diabetes. Assessment of diabetes status after surgery was classified into four categories according to the change in hypoglycemic agents after surgery and present status of T2DM: resolution, improvement, same, and worse.
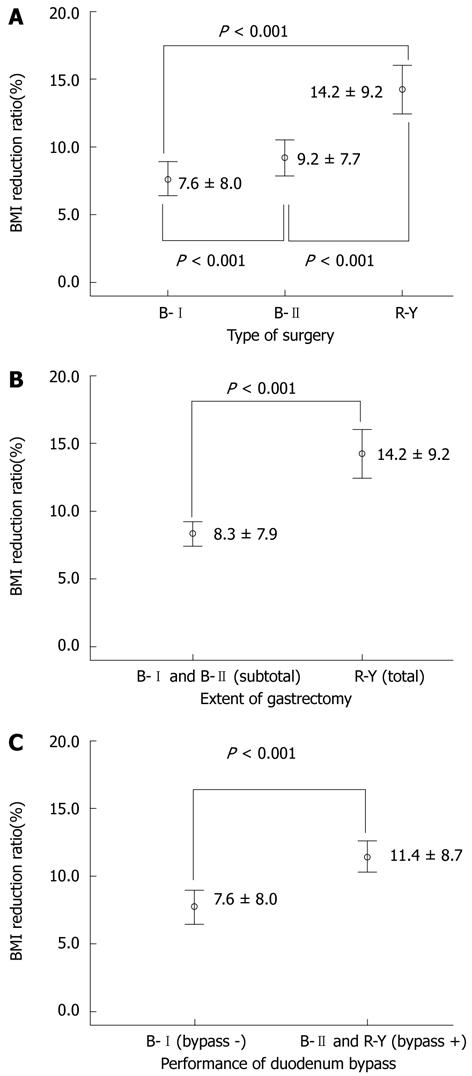
RESULTS: The mean follow-up duration was 33.7 mo (± 20.6 mo), preoperative BMI was 24.7 kg/m2 (± 3.0 kg/m2), and BMI reduction ratio was 9.8% (± 8.6%). After surgery, T2DM was cured in 58 patients (15.1%) and was improved in 117 patients (30.4%). According to the type of surgery, the BMI reduction ratio was significantly higher in the total gastrectomy and Roux-en-Y reconstruction group [14.2% ± 9.2% vs 9.2% ± 7.7% (Billroth II group), P < 0.001] and significantly lower in the subtotal gastrectomy and Billroth I reconstruction group [7.6% ± 8.0%, 9.2% ± 7.7% (Billroth II group), P < 0.001]. The BMI reduction ratio, follow-up duration after surgery, type of surgery, extent of gastrectomy, and performance of duodenal bypass were significantly correlated to the course of T2DM (P < 0.05). The BMI reduction ratio was the most influential factor on T2DM status. In a subgroup analysis of patients with a BMI reduction ratio of 10% or less (n = 206), T2DM was cured in 15 (7.6%) patients and was improved in 57 (28.8%) patients after surgery, and only the duration of surgery was significantly correlated to T2DM status (P = 0.022).
CONCLUSION: The course of T2DM was significantly correlated to the BMI reduction ratio but not to the type of surgery without a significant change in BMI.
- Citation: Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol 2012; 18(1): 49-54
- URL: https://www.wjgnet.com/1007-9327/full/v18/i1/49.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i1.49
Gastrointestinal surgery has been established as the treatment of choice for patients with inadequately controlled type 2 diabetes mellitus (T2DM) and a body mass index (BMI) greater than or equal to 35 kg/m2[1]. However, indications for bariatric surgery in the treatment of T2DM are not clear. T2DM is improved by calorie restriction and weight loss, which can be aided by bariatric surgery.
Recently, it was reported that bariatric surgery to treat morbid obesity along with T2DM resulted in amelioration of T2DM, including weight loss[2-6]. In addition, a non-randomized, comparative Swedish Obesity Study showed that regaining weight led to recurrence of diabetes through long-term follow-up[7]. However, bariatric surgery may improve glycemic control in T2DM within days, even before considerable weight loss occurs. The exact mechanism for the dramatic effect of obesity surgery on T2DM remains unknown. T2DM has been approached from a new angle as an intestinal disease, which is increasingly viewed as operable[8-11].
These findings prompted clinicians to extend indications for bariatric surgery to non-obese patients with T2DM. In the 1950s and 60s, amelioration of diabetes following subtotal gastrectomy was reported[12,13]. Since then, there have been few reports with inconsistent results in this area[14,15].
In this study, we compared the effects of gastrointestinal, anatomical rearrangements on diabetes after surgery for gastric cancer.
Between May 2003 and September 2009, 6848 patients with gastric cancer underwent gastrectomy at Severance Hospital and Gangnam Severance Hospital, the Yonsei University College of Medicine. Of these patients, 576 had diabetes; the diagnosis of diabetes was based on the criteria of the American Diabetes Association and the World Health Organization. We excluded 81 patients who expired or had cancer recurrence and 92 patients who could not be surveyed, leaving 403 patients to be enrolled for this study. To exclude type 1 diabetes, we reviewed patient medical records and interviewed these individuals about their type and history of diabetes, but no patients were diagnosed with or suspected to have type 1 diabetes. Data were collected by a review of medical records and interviews via telephone. The following factors were statistically analyzed: gender, age, type of surgery, preoperative BMI, current BMI, BMI reduction ratio, preoperative insulin or oral diabetic medicine requirement, follow-up duration, and current state of diabetes. “Resolution” was defined as a case where the patient became euglycemic or an HbA1c level was maintained below 6% without the use of diabetes medication after surgery. “Improvement” was defined as a case where the patient showed a better fasting glucose level and either used a lower dose of hypoglycemic agents or converted to oral hypoglycemic agents from insulin, compared with preoperative diabetic control. “Same” or “worse” was defined as a case in which no change occurred (“same”) or an aggravation in fasting glucose and medication after surgery (“worse”) was observed. Patients were divided into three groups by type of surgery: gastroduodenostomy after subtotal gastrectomy [Billroth I (B-I) group], gastrojejunostomy after subtotal gastrectomy [Billroth II (B-II) group], and Roux-en-Y esophagojejunostomy after total gastrectomy (R-Y group).
SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, United States) was used for all statistical analyses. All data were analyzed using the paired sample t test, analysis of variance, and ordinal logistic regression analysis. A P value of less than 0.05 was considered significant.
This study was approved by the Institutional Review Board of Gangnam Severance Hospital (approval number 3-2020-0259).
The characteristics of the subjects are summarized in Table 1. Of 403 patients, 58 patients (15.1%) were euglycemic without medication, and 117 patients (30.4%) showed improved metabolic conditions but were still taking medication. In contrast, 172 patients (44.7%) did not change their medication for diabetes, and 38 patients (9.9%) had aggravated conditions (Table 1).
| Gender | Female | 96 (23.8) |
| Male | 307 (76.2) | |
| Age at OP (yr) | 63.8 ± 7.6 | |
| OP type | B-I | 165 (40.9) |
| B-II | 134 (33.3) | |
| R-Y | 104 (25.8) | |
| Extent of gastrectomy | Subtotal (B-I, B-II) | 299 (74.2) |
| Total (R-Y) | 104 (25.8) | |
| Duodenal bypass | Bypass – (B-I) | 165 (40.9) |
| Bypass + (B-II, R-Y) | 238 (59.1) | |
| Pre-OP BMI (kg/m2) | 24.7 ± 3.0 (18.4-39.9) | |
| Current BMI (kg/m2) | 22.2 ± 2.9 (13.5-35.3) | |
| BMI reduction ratio1 (%) | 9.8 ± 8.6 | |
| Pre-OP | Oral diabetic agent | 368 (91.3) |
| diabetic therapy | Insulin | 35 (8.7) |
| F/U duration (mo) | 33.7 ± 20.6 (5.5-81.8) | |
| Diabetes course | Worse | 38 (9.9) |
| Same | 172 (44.7) | |
| Improvement | 117 (30.4) | |
| Resolution | 58 (15.1) |
Univariate analyses were carried out to determine the impact of factors contributing to the amelioration of diabetes. The course of diabetes after surgery was significantly different between the R-Y and B-I groups (P = 0.001) and between the R-Y and B-II groups (P = 0.006), but not between the B-I and B-II groups (P = 0.161). We categorized patients into two groups by the extent of gastrectomy. Diabetes was significantly improved in the total gastrectomy group compared with the subtotal gastrectomy group (P < 0.001) All patients were then categorized into two groups by the presence of duodenal bypass. Patients who received duodenal bypass had significantly improved diabetes compared to those who did not (P = 0.002, Table 2). In addition, the course of diabetes was significantly correlated with current BMI and BMI reduction ratio (P = 0.038 and P < 0.001, respectively). Diabetes was improved immediately after surgery, gradually worsening over a longer term (P = 0.001, Table 2).
| Diabetes course | P value | |||||
| Worse | Same | Improvement | Resolution | |||
| Gender | Female | 8 (8.6) | 36 (38.7) | 34 (36.6) | 15 (16.1) | 0.180 |
| Male | 30 (10.3) | 136 (46.6) | 83 (28.4) | 43 (14.7) | ||
| Age at OP (yr) | 62.6 ± 7.6 | 64.2 ± 7.4 | 63.5 ± 7.4 | 62.5 ± 8.4 | 0.453 | |
| OP type | B-Ia | 20 (12.4) | 82 (50.9) | 42 (26.1) | 17 (10.6) | 0.001 |
| B-IIa | 10 (8.0) | 59 (47.2) | 42 (33.6) | 14 (11.2) | 0.006 | |
| R-Y | 8 (8.1) | 31 (31.3) | 33 (33.3) | 27 (27.3) | ||
| Extent of gastrectomy | B-I and B-II (subtotal) | 30 (10.5) | 141 (49.3) | 84 (29.4) | 31 (10.8) | < 0.001 |
| R-Y (total) | 8 (8.1) | 31 (31.3) | 33 (33.3) | 27 (27.3) | ||
| Duodenal bypass | B-I (bypass -) | 20 (12.4) | 82 (50.9) | 42 (26.1) | 17 (10.6) | 0.002 |
| B-II and R-Y (bypass +) | 18 (8.0) | 90 (40.2) | 75 (33.5) | 41 (18.3) | ||
| Pre-OP BMI (kg/m2) | 23.4 ± 2.5 | 24.9 ± 2.6 | 24.8 ± 3.5 | 24.9 ± 3.5 | 0.127 | |
| Current BMI (kg/m2) | 21.7 ± 2.7 | 22.7 ± 2.6 | 22.2 ± 3.2 | 21.2 ± 2.9 | 0.038 | |
| BMI reduction ratio1 (%) | 6.8 ± 10.2 | 8.5 ± 7.3 | 10.4 ± 8.7 | 14.4 ± 8.5 | < 0.001 | |
| Pre-OP diabetic | Oral diabetic agent | 38 (10.8) | 159 (45.3) | 99 (28.2) | 55 (15.7) | 0.147 |
| regimen | Insulin | 0 (0.0) | 13 (38.2) | 18 (52.9) | 3 (8.8) | |
| F/U duration (mo) | 36.4 ± 18.2 | 37.2 ± 21.6 | 30.0 ± 20.1 | 28.3 ± 17.8 | 0.001 | |
Ordinal logistic regression analysis was performed to determine significant factors affecting the course of diabetes. Because surgery type, extent of gastrectomy, and duodenal bypass were similar factors, when the three factors were entered simultaneously in multivariate analysis, all were insignificant. Therefore, we performed multivariate analysis three times according to the three types of surgery. In all three multivariate analyses, the BMI reduction ratio and follow-up duration were significantly related to amelioration of diabetes, but current BMI was not. Moreover, the absolute value of contribution degree of the BMI reduction ratio was greatest among significant factors, so the BMI reduction ratio was found to have the biggest impact on diabetic status after three multivariate analyses (Table 3). Performance of B-I versus R-Y was a significant factor, but performance of B-II versus R-Y was not. We used the extent of gastrectomy or duodenal bypass as a covariate, and these factors were also significant.
| P value | B-estimation | SE | Contribution degree | |
| Current BMI (kg/m2) | 0.454 | 0.028 | 0.037 | |
| BMI reduction ratio1 (%) | < 0.001 | 0.051 | 0.013 | 3.948 |
| F/U duration (mo) | 0.007 | -0.013 | 0.005 | -2.703 |
| OP type | ||||
| B-I | 0.006 | -0.714 | 0.258 | -2.770 |
| B-II | 0.070 | -0.473 | 0.262 | |
| R-Y | ||||
| Current BMI (kg/m2) | 0.478 | 0.026 | 0.037 | |
| BMI reduction ratio1 (%) | < 0.001 | 0.052 | 0.013 | 4.031 |
| F/U duration (mo) | 0.006 | -0.013 | 0.005 | -2.727 |
| Extent of gastrectomy | ||||
| Subtotal (B-I, B-II) | 0.011 | -0.597 | 0.234 | -2.553 |
| Total (R-Y) | ||||
| Current BMI (kg/m2) | 0.657 | 0.016 | 0.037 | |
| BMI reduction ratio1(%) | < 0.001 | 0.054 | 0.013 | 4.181 |
| F/U duration (mo) | 0.005 | -0.013 | 0.005 | -2.811 |
| Duodenal bypass | ||||
| Bypass – (B-I) | 0.039 | -0.416 | 0.202 | -2.061 |
| Bypass + (B-II, R-Y) | ||||
Based on these results, we investigated the association between weight change and type of surgery. There was a significant decrease in BMI in the R-Y group, compared with the B-I and B-II groups (Figure 1A). There was also a significant difference in the BMI reduction ratio between the B-I and B-II groups. The total gastrectomy group showed significant weight loss compared with the subtotal gastrectomy group (P < 0.001, Figure 1B), and duodenal bypass lost significantly more weight than the non-duodenal bypass group (P < 0.001, Figure 1C).
These results suggest that the main factor influencing diabetes improvement might be weight loss instead of type of surgery. Therefore, we performed subgroup analysis of the patients whose BMI reduction ratio was less than 10% of the preoperative level. The mean BMI reduction ratio was 3.4% (± 5.4%; Table 4). In univariate analysis, type of surgery was not a significant factor for the amelioration of diabetes (Table 5). However, in this subgroup of 203 patients, diabetes was resolved without medication in 15 patients (7.6%), and 57 patients (36.4%) improved without significant weight loss.
| Gender | Female | 38 (18.4) |
| Male | 168 (81.6) | |
| Age at OP (yr) | ||
| OP type | B-I | 105 (51.0) |
| B-II | 68 (33.0) | |
| R-Y | 33 (16.0) | |
| Extent of gastrectomy | Subtotal (B-I, B-II) | 173 (84.0) |
| Total (R-Y) | 33 (16.0) | |
| Duodenal bypass | Bypass – (B-I) | 105 (51.0) |
| Bypass + (B-II, R-Y) | 101 (49.0) | |
| Pre-OP BMI (kg/m2) | 23.8 ± 2.8 (18.4-32.6) | |
| Current BMI (kg/m2) | 23.0 ± 2.7 (21.8-32.5) | |
| BMI reduction ratio1 (%) | 3.4 ± 5.4 | |
| Pre-OP diabetic regimen | Oral diabetic agent | 186 (90.3) |
| Insulin | 20 (9.7) | |
| F/U duration (mo) | 35.7 ± 20.7 (5.5-81.1) | |
| Diabetes course | Worse | 24 (12.1) |
| Same | 102 (51.5) | |
| Improvement | 57 (28.8) | |
| Resolution | 15 (7.6) |
| Diabetes course | P value | |||||
| Worse | Same | Improvement | Remission | |||
| Gender | Female | 3 (8.1) | 17 (45.9) | 14 (37.8) | 3 (8.1) | 0.190 |
| Male | 21 (13.0) | 85 (52.8) | 43 (26.7) | 12 (7.5) | ||
| Age at OP (yr) | 61.9 ± 8.1 | 63.9 ± 8.0 | 63.8 ± 7.5 | 61.1 ± 9.3 | 0.976 | |
| OP type | B-Ia | 14 (13.5) | 56 (53.8) | 27 (26.0) | 7 (6.7) | 0.076 |
| B-IIa | 7 (11.3) | 33 (53.2) | 18 (29.0) | 4 (6.5) | 0.181 | |
| R-Y | 3 (9.4) | 13 (40.6) | 12 (37.5) | 4 (12.5) | ||
| Extent of gastrectomy | Subtotal (B-I, B-II) | 21 (12.7) | 89 (53.6) | 45 (27.1) | 11 (6.6) | 0.084 |
| Total (R-Y) | 3 (9.4) | 13 (40.6) | 12 (37.5) | 4 (12.5) | ||
| Duodenal bypass | Bypass – (B-I) | 14 (13.5) | 56 (53.8) | 27 (26.0) | 7 (6.7) | 0.250 |
| Bypass + (B-II, R-Y) | 10 (10.6) | 46 (48.9) | 30 (31.9) | 8 (8.5) | ||
| Pre-OP BMI (kg/m2) | 22.7 ± 2.3 | 24.3 ± 2.7 | 23.8 ± 3.0 | 22.2 ± 2.8 | 0.615 | |
| Current BMI (kg/m2) | 22.3 ± 2.2 | 23.4 ± 2.6 | 23.0 ± 3.1 | 21.3 ± 2.6 | 0.310 | |
| BMI reduction ratio1 (%) | 1.5 ± 6.6 | 3.7 ± 4.5 | 3.6 ± 5.9 | 4.2 ± 4.6 | 0.183 | |
| Pre-OP | Oral diabetic agent | 24 (13.5) | 93 (52.2) | 47 (26.4) | 14 (7.9) | 0.080 |
| diabetic | ||||||
| regimen | Insulin | 0 (0.0) | 9 (45.0) | 10 (50.0) | 1 (5.0) | |
| F/U duration (mo) | 37.7 ± 17.6 | 38.8 ± 21.7 | 30.7 ± 20.7 | 28.7 ± 14.1 | 0.022 | |
This study was conducted in a retrospective manner. Therefore, the type of diabetes was not evaluated by laboratory testing but by reviewing medical records and interviewing. We interviewed all of the enrolled patients and asked about the type and history of diabetes to exclude type 1 diabetes. There were no patients who were diagnosed with or suspected to have type 1 diabetes. Furthermore, in Korea, the incidence of type 1 diabetes is very low, reported as approximately 0.6-2.2/100 000[16-18]. Considering that type 1 diabetes is extremely rare in Korea and the mean age of the study population was 63.8 years, the results of this study showing that there were no cases of type 1 diabetes in 6848 patients of the study population can be thought acceptable.
Possible mechanisms through which metabolic surgery has led to the resolution of T2DM are a decrease in calorie intake, weight loss, and a rearranged gastrointestinal track, which could modulate hormones that affect glucose metabolism, insulin sensitivity, and beta pancreatic cell proliferation[19]. Several reports support a positive role for duodenojejunal exclusion and/or duodenal bypass for T2DM resolution after bariatric surgery in obese patients[20].
However, it is still unclear whether these effects extend to non-obese patients. One report stressed that gastrectomy and short Roux-en Y reconstruction in non-obese T2DM patients correlate with remission of diabetes in 65% of patients[14]. In that study, the preoperative BMI was 29.1 and the postoperative BMI was 24.6 kg/m2; such significant weight loss might explain the relatively high rate of T2DM resolution after gastrectomy. Another study reported a resolution rate of 38.1% after gastrectomy with Roux-en Y gastrojejunostomy, which is similar to our results[15]. Early delivery of partially digested foods to the ileum and discrete enteroendocrine cells in the mucosa are suggested to release hormones that contribute to the ileal brake[21]. However, we could not evaluate this point due to procedural limitations.
One of the mainstream T2DM treatments is reduction of calorie intake; restrictive bariatric surgery effectively controls T2DM in obese patients[2]. In this study, univariate and multivariate analyses showed that duodenal bypass and/or duodenojejunal exclusion groups demonstrated a high rate of T2DM resolution compared to other procedures. However, for patients who lost less than 10% of their preoperative weight, the type of surgery was not significantly important for T2DM resolution. These results suggest that the usual procedures after gastrectomy for gastric cancer are ineffective to control T2DM directly. In addition, most gastrectomized patients for gastric cancer are not pathologically obese, and reconstruction should not induce severe weight loss.
Interestingly, diabetes worsened after improvements were observed for a short time after surgery. The limitation of this study, which is retrospective and cross-sectional analysis, should be considered. Moreover, most gastric cancer patients are cautious about maintaining their weight for good nutrition. Therefore, it is possible that weight loss immediately after surgery improves T2DM, and as the patient regains weight, the condition deteriorates. In meta-analysis, the proportion of patients with resolution or improvement was not significantly different at time points less than 2 years or 2 years or more after surgery, although it slightly decreased over time[2].
Several studies demonstrated that T2DM could be resolved after metabolic surgery, irrespective of weight loss, via a modification in the enteroinsular axis[2,20,21]. Based on these findings, we tried to establish a new procedure for the resolution of T2DM in non-obese patients. Unfortunately, routine reconstruction surgery after gastrectomy could not control diabetes in non-obese patients, and the main mechanism for gastrectomy-induced resolution of T2DM is possibly weight loss, instead of the type of surgery itself. Thus, a more sophisticated procedure should be developed and prospectively evaluated for its effectiveness in controlling T2DM in gastric cancer patients after gastrectomy.
It has been frequently reported that bariatric surgery for morbid obesity along with type II diabetes mellitus (T2DM) results in amelioration of T2DM, including weight loss. Furthermore, in some reports, bariatric surgery may improve glycemic control in T2DM within days, even before considerable weight loss occurs. These findings prompted clinicians to consider T2DM as an operable gastrointestinal disease and extend indications for bariatric surgery to non-obese patients with T2DM. However, the application of surgery for non-obese T2DM patients has considerable risks. In Korea, many cases of gastric cancer have been performed by gastric cancer surgery. In this study, the effects of gastrointestinal, anatomical rearrangements on diabetes were compared after surgery for gastric cancer.
This is a retrospective but very large-scale study. The authors interviewed all of the enrolled subjects via telephone.
In the 1950s and 60s, amelioration of diabetes following subtotal gastrectomy was reported. Since then, there have been few reports in this area with inconsistent results. In those reports, the numbers of cases were very small. In this study, 403 patients were enrolled, and we evaluated the factors affecting the course of diabetes. The authors demonstrated that 15% of T2DM was cured after gastrectomy with conventional reconstruction. The extent of gastrectomy, type of reconstruction, and change in body mass index (BMI) were related to the course of diabetes. Among these factors, the change in BMI was the strongest. In the subgroup analysis of small BMI changes, the extent of gastrectomy and the type of reconstruction were not statistically related to the course of diabetes, which did not support the foregut hypothesis.
The results of this study can be used as reference data for further study. In other words, in some study of innovative treatment for diabetes in gastric cancer patients, the results could be used for the calculation of the number of subjects required and provide reference data for comparison. In addition, duodenal bypass itself is not a critical factor in metabolic surgery.
The term duodenal bypass means a condition in which ingested food material does not traverse the duodenum. In both Billroth II reconstruction after subtotal gastrectomy and Roux-en-Y reconstruction after total gastrectomy, ingested food materials go directly to the jejunum. Metabolic surgery is a field of surgery that attempts to treat metabolic syndrome, including diabetes. Hormonal changes after metabolic surgery are thought to be important. Both the hindgut hypothesis (the quick transit of nutrients to the distal bowel is important) and the foregut hypothesis (the exclusion of the proximal small intestine induces resolution of T2DM) have been proposed.
This paper summarized the effect of gastrectomy on T2DM in a large number of patients.
Peer reviewer: Kazuma Fujimoto, Professor, Department of Internal Medicine, Saga Medical School, Nabeshima, Saga 849-8501, Japan
S- Editor Tian L L- Editor Rutherford A E- Editor Zhang DN
| 1. | Rubino F, Kaplan LM, Schauer PR, Cummings DE. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1816] [Cited by in F6Publishing: 1664] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 3. | Lee WJ, Chong K, Lee YC, Ser KH, Chen SC, Chen JC, Peng WP, Chen CM. Effects of obesity surgery on type 2 diabetes mellitus Asian patients. World J Surg. 2009;33:1895-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Greenway SE, Greenway FL, Klein S. Effects of obesity surgery on non-insulin-dependent diabetes mellitus. Arch Surg. 2002;137:1109-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5073] [Cited by in F6Publishing: 4544] [Article Influence: 227.2] [Reference Citation Analysis (0)] |
| 6. | Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467-484; discussion 484-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3301] [Cited by in F6Publishing: 2914] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 8. | Hickey MS, Pories WJ, MacDonald KG, Cory KA, Dohm GL, Swanson MS, Israel RG, Barakat HA, Considine RV, Caro JF. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg. 1998;227:637-643; discussion 643-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 267] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31 Suppl 2:S290-S296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis. 2008;18:574-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Friedman MN, Sancetta AJ, Magovern GJ. The amelioration of diabetes mellitus following subtotal gastrectomy. Surg Gynecol Obstet. 1955;100:201-204. [PubMed] [Cited in This Article: ] |
| 13. | Angervall L, Dotevall G, Tillander H. Amelioration of diabetes mellitus following gastric resection. Acta Med Scand. 1961;169:743-748. [PubMed] [Cited in This Article: ] |
| 14. | Lanzarini E, Csendes A, Lembach H, Molina J, Gutiérrez L, Silva J. Evolution of type 2 diabetes mellitus in non morbid obese gastrectomized patients with Roux en-Y reconstruction: retrospective study. World J Surg. 2010;34:2098-2102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Yang J, Li C, Liu H, Gu H, Chen P, Liu B. Effects of subtotal gastrectomy and Roux-en-Y gastrojejunostomy on the clinical outcome of type 2 diabetes mellitus. J Surg Res. 2010;164:e67-e71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Park Y. Why is type 1 diabetes uncommon in Asia? Ann N Y Acad Sci. 2006;1079:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia. 1993;36:883-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 266] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Ahn KJ. Is Diabetes in Korea Different? J Korean Med Association. 2005;48:8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Villamizar N, Pryor AD. Safety, effectiveness, and cost effectiveness of metabolic surgery in the treatment of type 2 diabetes mellitus. J Obes. 2011;2011:790683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Rubino F, R'bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6:102-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Spector D, Shikora S. Neuro-modulation and bariatric surgery for type 2 diabetes mellitus. Int J Clin Pract Suppl. 2010;53-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |