Published online Jul 21, 2012. doi: 10.3748/wjg.v18.i27.3623
Revised: April 23, 2012
Accepted: April 27, 2012
Published online: July 21, 2012
The incidence of colonic diverticulosis with or without diverticulitis has increased in the Japanese population due to the modernization of food and aging. The rate of diverticulitis in colon diverticulosis ranges from 8.1% to 9.6%. However, few cases of stenosis due to diverticulitis have been reported. These reports suggest that the differentiation between sigmoid diverticulitis and colon cancer is difficult. This report describes two cases of colon stenosis due to diverticulitis that were difficult to differentiate from colon cancer. Case 1 was a 70-year-old woman with narrowed stools for 1 month who underwent colonofiberscopy (CFS). CFS revealed a diverticulum and circumferential stenosis in the sigmoid colon. Barium enema revealed a marked, hourglass-shaped, 2-cm circumferential stenosis in the sigmoid colon. Fluorodeoxyglucose (FDG)-positron emission tomography computed tomography (CT) revealed an increased FDG uptake at the affected portion of the sigmoid colon. Sigmoid colon cancer was suspected, and laparoscopic sigmoidectomy was performed. Pathological examination demonstrated active inflammation with no evidence of malignancy. Case 2 was a 50-year-old man who presented to a nearby clinic with reduced stool output despite the urge to defecate. CFS detected severe stenosis in the sigmoid colon approximately 25 cm from the dentate line. Contrast-enhanced abdominal CT revealed multiple diverticula, wall thickening, and swelling of the lymph nodes around the peritoneal aorta and the inferior mesenteric artery. A partial sigmoidectomy was performed. Pathological examination of the resected specimen revealed no changes in the mucosal epithelial surface, but a marked infiltration of inflammatory cells was observed.
- Citation: Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Masaki T. Difficulty in differentiating two cases of sigmoid stenosis by diverticulitis from cancer. World J Gastroenterol 2012; 18(27): 3623-3626
- URL: https://www.wjgnet.com/1007-9327/full/v18/i27/3623.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i27.3623
The incidence of colonic diverticulosis with or without diverticulitis has increased in the Japanese population due to the modernization of food and aging[1].
The rate of diverticulitis in colon diverticulosis ranges from 8.1% to 9.6%. However, few cases of stenosis due to diverticulitis have been reported. These reports suggest that the differentiation between sigmoid diverticulitis and colon cancer is difficult.
No causal relationship between colonic diverticulosis and colon cancer has been established[2], but a significant correlation between these two conditions has been identified[3]. Barium enema[4], positron emission tomography- computed tomography (PET-CT), and magnetic resonance imaging-diffusion weighted imaging (MRI-DWI) are useful for the differential diagnosis between inflammation and cancer in the large intestine. This report describes and discusses two cases of sigmoid stenosis due to sigmoid diverticulitis that were difficult to differentiate from colon cancer using colonofiberscopy (CFS), barium enema, and PET-CT.
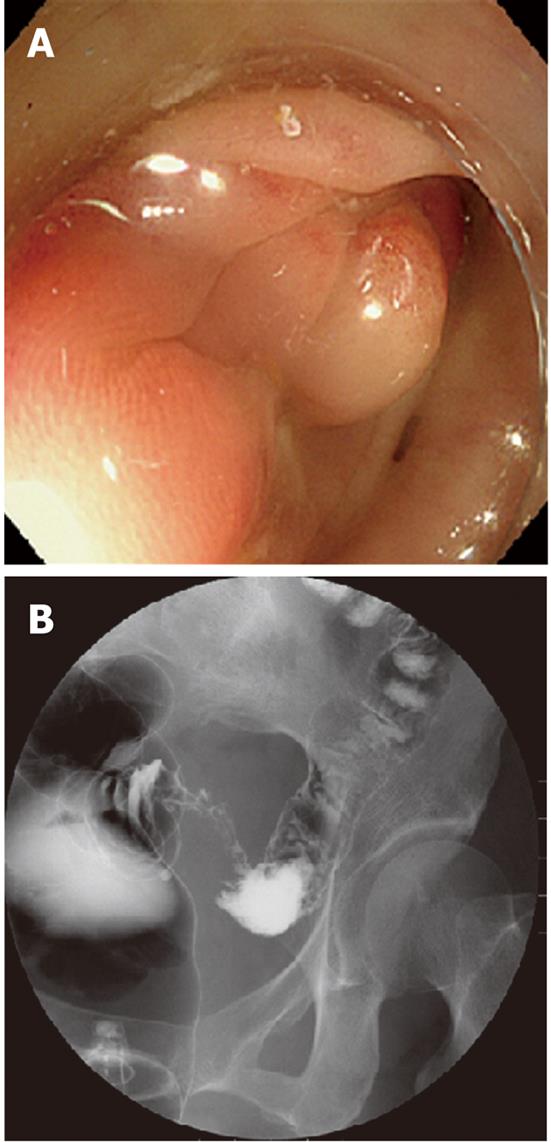
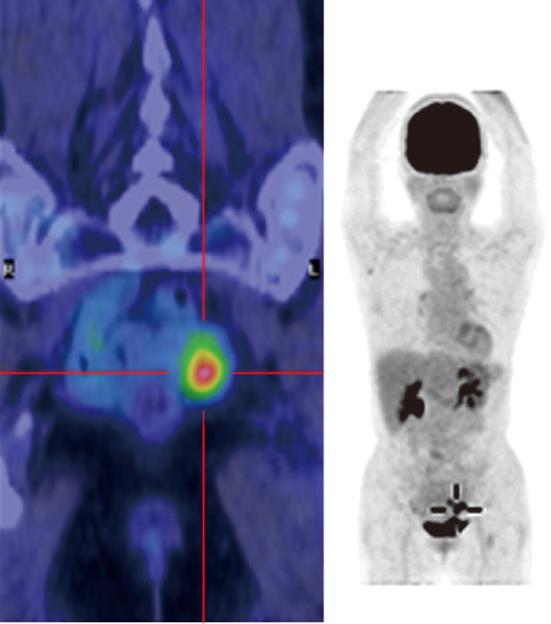
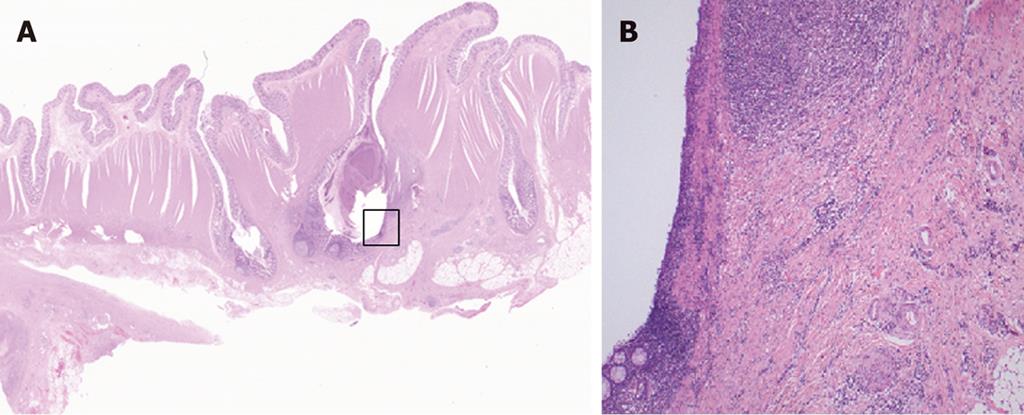
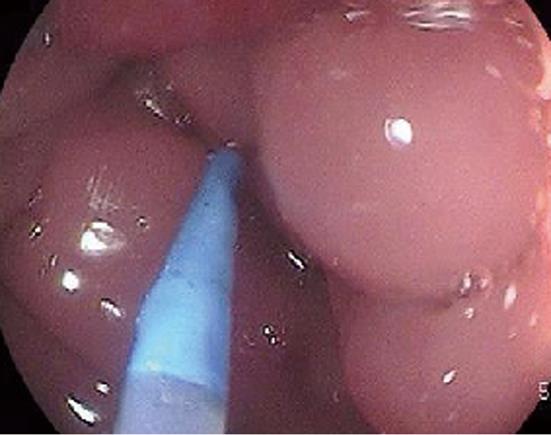
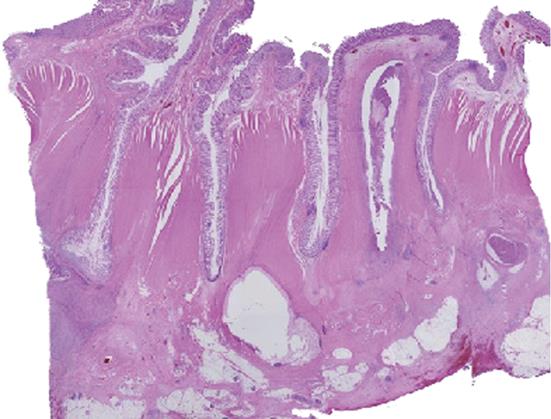
Case 1 was a 70-year-old woman with narrowed stools for 1 month who underwent CFS of the lower gastrointestinal tract at a nearby clinic. CFS revealed a diverticulum and circumferential stenosis in the sigmoid colon. The mucosa at the stenotic site was discolored, and the scope could not be advanced beyond the stenosis (Figure 1A). Biopsy showed no evidence of malignancy, and laboratory tests revealed no abnormal findings, including tumor markers [carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9)]. Contrast-enhanced abdominal CT revealed a marked thickening in the sigmoid colon wall. Barium enema revealed a marked, hourglass-shaped, 2-cm circumferential stenosis in the sigmoid colon (Figure 1B). Fluorodeoxyglucose [18F] (FDG)-PET CT revealed an increased FDG uptake at the affected portion of the sigmoid colon (Figure 2). Sigmoid colon cancer was suspected, and laparoscopic sigmoidectomy was performed. Pathological examination of the resected specimen revealed active inflammation characterized by a marked thickening of the muscularis propria, fibrous thickening of interstitial tissue, and a marked infiltration of inflammatory cells with no evidence of malignancy (Figure 3). The patient was diagnosed with inflammatory thickening and stenosis of the sigmoid colon due to sigmoid diverticulitis. The patient had a favorable postoperative course, and she was discharged from the hospital.
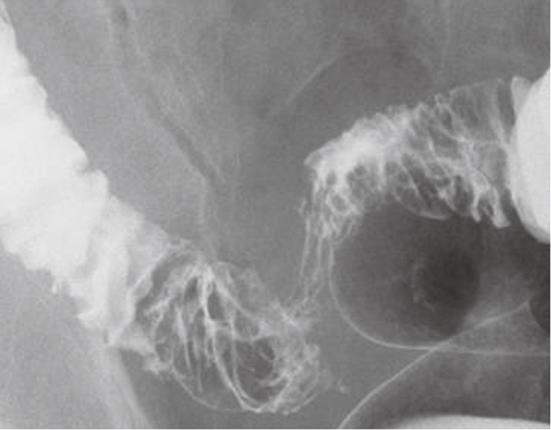
Case 2 was a 50-year-old man who presented to a nearby clinic with reduced stool output despite the urge to defecate. Abdominal X-ray revealed mild gas accumulation from the ascending colon to the transverse colon but no evidence of ileus. The patient was placed under observation; however, six months later, he was admitted to our hospital with a lack of bowel movement and lower abdominal pain with increased body temperature. Laboratory tests after admission indicated severe inflammation, including a white blood cell count of 13 520 cells/μL and C-reactive protein of 13.5 mg/dL but no abnormal tumor markers (CEA and CA 19-9). Barium enema revealed a segmental stenosis of approximately 10 cm, mucosal wall thickening, and cobblestone-like mucosa in the sigmoid colon (Figure 4). CFS detected severe stenosis in the sigmoid colon approximately 25 cm from the dentate line, and the scope could not be advanced further. Mucosal surfaces in the visible range were edematous and red with a partial polyposis-like appearance (Figure 5). Biopsy suggested the presence of inflammation only. Contrast-enhanced abdominal CT revealed multiple diverticula, wall thickening, an increase in surrounding fat tissue, and swelling of the lymph nodes around the peritoneal aorta and the inferior mesenteric artery. FDG-PET also revealed wall thickening, lumen narrowing, and abnormal FDG uptake in the affected portion of the sigmoid colon. A marked increase in FDG uptake was also observed in the surrounding lymph nodes, which suggested a metastasis of the sigmoid cancer to para-aortic lymph nodes (T3N1M1). Partial sigmoidectomy was performed. Pathological examination of the resected specimen revealed no changes in the mucosal epithelial surface, but a marked infiltration of inflammatory cells was observed (Figure 6). The patient was diagnosed with sigmoid diverticulitis, which led to circumferential stenosis due to severe and chronic inflammation.
Colonic diverticulosis is generally asymptomatic, but it can lead to diverticulitis, diverticular hemorrhage, and other complications in rare cases. A colonic diverticulum is a false diverticulum without muscularis propria, and the occurrence of diverticulitis leads to the spread of inflammation beyond the intestine. Only 0.09% of patients with diverticulosis develop diverticulitis and subsequent stenosis, which occurs more frequently in the left colon[5].
The two cases in this study presented difficult preoperative differential diagnoses, which included benign lesions, such as intestinal tuberculosis and metastatic colon cancer, and malignancies with minimal tumor exposure on the mucosal surface, such as diffusely infiltrating colon cancer. However, no characteristic macroscopic findings were obtained, and biopsies did not provide a definitive diagnosis.
Benign lesions such as intestinal tuberculosis do not require immediate diagnosis because these lesions are post-healing changes. However, malignancies such as colon cancer require early diagnosis. The criteria for the differentiation of diverticulitis and cancer using barium enema X-ray results have been reported by Berman and Kinsner[4]. The cases in this report could have been diagnosed as malignant using these criteria due to the stenosis length, the presence of an hourglass-shaped circumferential stenosis, clear borders and steep margins, despite their almost normal mucosal appearances. PET-CT was performed to differentiate between benign and malignant lesions, and an increased uptake was observed in both cases.
A delayed PET-CT reveals an increased uptake during active inflammation, which is a malignant pattern. However, the PET-CT of lung lesions reveals malignant lesions with higher maximum standardized uptake value levels than inflammatory lesions[6]. A greater increase in F-FDG uptake at 60 s has been demonstrated in malignant tumors compared to inflammatory lesions in animals[7].
Cut-off value for a malignant diagnosis using FDG has not been established. Colon cancer arising from colonic diverticulum has been reported previously, but Haubrich et al[2] demonstrated no correlation between colonic diverticulum and colon cancer. Conversely, a significantly higher prevalence of colon cancer in patients with and without multiple sigmoid diverticula was observed in a study conducted with a population of Japanese patients by Mihara et al[8]. Patients with colonic diverticulosis who develop colon cancer tend to develop diverticulitis at the same site[9]. Therefore, a diagnosis of colon cancer should be considered in a patient with low levels of FDG uptake on PET-CT.
A lower false-positive rate for the diagnosis of inflammatory lesions has been demonstrated using MRI-DWI compared to PET in animals, but the sensitivity and specificity of MRI-DWI were similar to PET-CT[10]. These results suggest that MRI-DWI is a useful diagnostic tool when the differentiation between colon cancer and inflammation is difficult.
Peer reviewer: Dr. Xiaoyun Liao, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Avenue, Room JF-208E, Boston, MA 02215, United States
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450-454. [PubMed] [Cited in This Article: ] |
| 2. | McCallum A, Eastwood MA, Smith AN, Fulton PM. Colonic diverticulosis in patients with colorectal cancer and in controls. Scand J Gastroenterol. 1988;23:284-286. [PubMed] [Cited in This Article: ] |
| 3. | Localio SA, Stahl WM. Diverticular disease of the alimentary tract. I. The colon. Curr Probl Surg. 1967;1-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Berman PM, Kirsner JB. Current knowledge of diverticular disease of the colon. Am J Dig Dis. 1972;17:741-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Sugihara K, Muto T, Morioka Y, Asano A, Yamamoto T. Diverticular disease of the colon in Japan. A review of 615 cases. Dis Colon Rectum. 1984;27:531-537. [PubMed] [Cited in This Article: ] |
| 6. | Yang SN, Liang JA, Lin FJ, Kwan AS, Kao CH, Shen YY. Differentiating benign and malignant pulmonary lesions with FDG-PET. Anticancer Res. 2001;21:4153-4157. [PubMed] [Cited in This Article: ] |
| 7. | Liu P, Huang G, Dong S, Wan L. Kinetic analysis of experimental rabbit tumour and inflammation model with 18F-FDG PET/CT. Nuklearmedizin. 2009;48:153-158. [PubMed] [Cited in This Article: ] |
| 8. | Mihara O, Miyatake K, Ariyoshi Y, Endoh T, Kido C. Complication of colonic carcinoma in multiple diverticulosis involved in the sigmoid colon. Stomach and Intestine (Tokyo). 1979;14:239-244. [Cited in This Article: ] |
| 9. | Stavorovsky M, Finkelstein T. Colonic cancer and associated diverticulitis. Int Surg. 1979;64:49-53. [PubMed] [Cited in This Article: ] |
| 10. | Mori T, Nomori H, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, Yamashita Y. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol. 2008;3:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |