Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4379
Revised: August 7, 2012
Accepted: August 14, 2012
Published online: August 28, 2012
AIM: To study the prevalence of functional dyspepsia (FD) (Rome III criteria) across eating disorders (ED), obese patients, constitutional thinner and healthy volunteers.
METHODS: Twenty patients affected by anorexia nervosa, 6 affected by bulimia nervosa, 10 affected by ED not otherwise specified according to diagnostic and statistical manual of mental disorders, 4th edition, nine constitutional thinner subjects and, thirty-two obese patients were recruited from an outpatients clinic devoted to eating behavior disorders. Twenty-two healthy volunteers matched for age and gender were enrolled as healthy controls. All participants underwent a careful clinical examination. Demographic and anthropometric characteristics were obtained from a structured questionnaires. The presence of FD and, its subgroups, epigastric pain syndrome and postprandial distress syndrome (PDS) were diagnosed according to Rome III criteria. The intensity-frequency score of broader dyspeptic symptoms such as early satiety, epigastric fullness, epigastric pain, epigastric burning, epigastric pressure, belching, nausea and vomiting were studied by a standardized questionnaire (0-6). Analysis of variance and post-hoc Sheffè tests were used for comparisons.
RESULTS: 90% of patients affected by anorexia nervosa, 83.3% of patients affected by bulimia nervosa, 90% of patients affected by ED not otherwise specified, 55.6% of constitutionally thin subjects and 18.2% healthy volunteers met the Postprandial Distress Syndrome Criteria (χ2, P < 0.001). Only one bulimic patient met the epigastric pain syndrome diagnosis. Postprandial fullness intensity-frequency score was significantly higher in anorexia nervosa, bulimia nervosa and ED not otherwise specified groups compared to the score calculated in the constitutional thinner group (4.15 ± 2.08 vs 1.44 ± 2.35, P = 0.003; 5.00 ± 2.45 vs 1.44 ± 2.35, P = 0.003; 4.10 ± 2.23 vs 1.44 ± 2.35, P = 0.002, respectively), the obese group (4.15 ± 2.08 vs 0.00 ± 0.00, P < 0.001; 5.00 ± 2.45 vs 0.00 ± 0.00, P < 0.001; 4.10 ± 2.23 vs 0.00 ± 0.00, P < 0.001, respectively) and healthy volunteers (4.15 ± 2.08 vs 0.36 ± 0.79, P < 0.001; 5.00 ± 2.45 vs 0.36 ± 0.79, P < 0.001; 4.10 ± 2.23 vs 0.36 ± 0.79, P < 0.001, respectively). Early satiety intensity-frequency score was prominent in anorectic patients compared to bulimic patients (3.85 ± 2.23 vs 1.17 ± 1.83, P = 0.015), obese patients (3.85 ± 2.23 vs 0.00 ± 0.00, P < 0.001) and healthy volunteers (3.85 ± 2.23 vs 0.05 ± 0.21, P < 0.001). Nausea and epigastric pressure were increased in bulimic and ED not otherwise specified patients. Specifically, nausea intensity-frequency-score was significantly higher in bulimia nervosa and ED not otherwise specified patients compared to anorectic patients (3.17 ± 2.56 vs 0.89 ± 1.66, P = 0.04; 2.70 ± 2.91 vs 0.89 ± 1.66, P = 0.05, respectively), constitutional thinner subjects (3.17 ± 2.56 vs 0.00 ± 0.00, P = 0.004; 2.70 ± 2.91 vs 0.00 ± 0.00, P = 0.005, respectively), obese patients (3.17 ± 2.56 vs 0.00 ± 0.00, P < 0.001; 3.17 ± 2.56 vs 0.00 ± 0.00, P < 0.001 respectively) and, healthy volunteers (3.17 ± 2.56 vs 0.17 ± 0.71, P = 0.002; 3.17 ± 2.56 vs 0.17 ± 0.71, P = 0.001, respectively). Epigastric pressure intensity-frequency score was significantly higher in bulimic and ED not otherwise specified patients compared to constitutional thin subjects (4.67 ± 2.42 vs 1.22 ± 1.72, P = 0.03; 4.20 ± 2.21 vs 1.22 ± 1.72, P = 0.03, respectively), obese patients (4.67 ± 2.42 vs 0.75 ± 1.32, P = 0.001; 4.20 ± 2.21 vs 0.75 ± 1.32, P < 0.001, respectively) and, healthy volunteers (4.67 ± 2.42 vs 0.67 ± 1.46, P = 0.001; 4.20 ± 2.21 vs 0.67 ± 1.46, P = 0.001, respectively). Vomiting was referred in 100% of bulimia nervosa patients, in 20% of ED not otherwise specified patients, in 15% of anorexia nervosa patients, in 22% of constitutional thinner subjects, and, in 5.6% healthy volunteers (χ2, P < 0.001).
CONCLUSION: PDS is common in eating disorders. Is it mandatory in outpatient gastroenterological clinics to investigate eating disorders in patients with PDS?
- Citation: Santonicola A, Siniscalchi M, Capone P, Gallotta S, Ciacci C, Iovino P. Prevalence of functional dyspepsia and its subgroups in patients with eating disorders. World J Gastroenterol 2012; 18(32): 4379-4385
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4379
Eating disorders (ED) are highly prevalent health problems in Western countries, especially in young women[1]. Although no consensus has been yet achieved in the definition of eating disorders[2], three main ED categories have been identified according to the diagnostic and statistical manual of mental disorders, 4th edition (DSM-IV)[3]: anorexia nervosa (AN), bulimia nervosa (BN), and eating disorders not otherwise specified (EDNOS). In ED patients there is a significant impairment of both physical health and psychosocial functioning[4]. Gastrointestinal (GI) symptoms are a common complaint in these patients. Boyd et al[5] interviewed 101 ED patients (44% AN, 22% BN, 34% EDNOS), using a standardized questionnaire to assess the presence of functional gastrointestinal disorders (FGIDs) such as irritable bowel syndrome (IBS), functional heartburn, functional abdominal bloating, functional constipation, functional dysphagia and functional anorectal pain disorder, showing that 98% of ED patients fulfilled the criteria for at least one FGID. A recent study demonstrated that 68.8% of ED patients met the Manning criteria for IBS[6]. However, it was suggested that the wide range of FGIDs found in ED were the result of the behavior-associated ED. In fact, these GI symptoms may persist even after the recovery from ED, especially in psychologically distressed patients[7]. However, the underlying mechanisms that link ED and GI symptoms remain to be elucidated[8].
It is a common occurrence that patients, before presenting to healthcare services with an ED, seek treatment for GI symptoms[9]. FGIDs induce high health-care utilization and negative impact on quality of life[10]. Dyspeptic symptoms are very common in the general population, with prevalence estimates ranging between 10% and 45%[11,12]. The results of prevalence studies are strongly influenced by the criteria used to define dyspepsia. Well-performed epidemiological studies have reported a prevalence of approximately 20%-25% in western countries[13,14], slightly higher in women, with a variable influence of age across studies.
Currently, an internationally accepted clinical standard (Rome III criteria) is extensively used to diagnoseFGIDs[15]. The Rome III Criteria were developed by a Committee that recommended the following pragmatic description of functional dyspepsia (FD) defined as the presence of symptoms thought to originate in the gastroduodenal region, in the absence of any organic, systemic, or metabolic disease that is likely to explain the symptoms.The specific symptoms needed to diagnose FD are: epigastric pain, epigastric burning, post-prandial fullness and early satiation. In addition, the Rome III consensus offers an umbrella definition for FD, and, furthermore, helps to distinguish whether patients report symptom aggravation after ingestion of a meal, meal-related dyspeptic symptoms, the so called postprandial distress syndrome (PDS) characterized by postprandial fullness and early satiation or meal-unrelated dyspeptic symptoms, the so called epigastric pain syndrome (EPS), characterized by epigastric pain and epigastric burning[16]. A distinction between meal-related and meal-unrelated symptoms might be pathophysiologically and clinically relevant to disclose differences across ED, and other groups of patients with different patterns of abnormal eating behavior such as obese patients (OB) and constitutional thinness subjects (CT) in comparison to healthy volunteers (HV).
Our primary aim was to study the prevalence of FD and its subgroups according to the Rome III criteria across ED in comparison to OB patients, CT subjects and HV. Secondary aims were the evaluation of the frequency-intensity score of broader dyspeptic symptoms such as early satiety, epigastric fullness, epigastric pain, epigastric burning, epigastric pressure, belching, nausea and vomiting in ED patients compared to the other groups of patients with different patterns of abnormal eating behavior.
Five groups of patients matched for age and gender were recruited from an outpatients clinic devoted to eating behavior disorders. The first group consisted of 20 patients (AN-group), the second group of 6 BN patients (BN-group), the third group of 10 EDNOS patients (EDNOS-group), the fourth group of 9 CT subjects (CT-group) and the last group of 32 OB patients (OB-group). Twenty-two HV were recruited among administrative and/or paramedical staff members and patients’ friends as the control healthy group (HV-group).
All patients and HV were interviewed to detect lifetime eating disorders in accordance with the criteria of the DSM-IV[3]. The DSM-IV criteria define anorexia nervosa as self-induced weight loss or refusal to maintain or gain weight normally, with resulting weight more than 15% below normal; intense fear of fatness or gaining weight, even though underweight; deep disturbance in body image; and reproductive hormone abnormality (for example, at least 3 mo of amenorrhea).
Bulimia nervosa is defined as recurrent episodes of binge eating (a large amount of food eaten quickly and privately with lack of control over eating) and recurrent inappropriate compensatory behaviour to prevent weight gain (self-induced vomiting; misuse of laxatives, diuretics, enemas, or other medications; fasting; excessive exercise) at least twice a week for at least 3 mo, and self-evaluation unduly influenced by body shape and weight.
EDNOS represents the third category of ED and involves milder versions of anorexia and bulimia nervosa that do not satisfy all the criteria (for example, a binge episode once a week or for less than 3 mo for bulimia nervosa; weight loss less than 15% for anorexia nervosa).
CT subjects were recruited among the patients evaluated for leanness, using the following inclusion criteria: severely underweight, but stable throughout the post-pubertal period, presence of physiological menstruations without estroprogestative treatment, and the desire for weight gain as the main reason for medical consultation, together with the exclusion of celiac disease, infectious diseases, cancer, or other consumptive diseases[17].
Obesity is defined if the body mass index (BMI) was ≥ 30 kg/m2 according to the National Institute of Health guidelines[18].
For each patient, demographic (age, smoking habits, alcohol intake) and anthropometric characteristics (weight, height and BMI) were collected.
All patients gave their written consent to participate into the study. The study, fully complied with the Declaration of Helsinki, and was approved by the Ethics Committee of the Ruggi d’Aragona Hospital AOU University of Salerno.
All participants underwent a standardized questionnaire testing the presence of FD according to Rome III criteria. The Rome III symptom questionnaire consisted of 18 questions and allowed the diagnosis of FD and its subgroups (PDS and EPS). The characteristic symptoms of PDS were bothersome postprandial fullness or early satiation and those of EPS were unexplained epigastric pain or burning[16]. The frequency for early satiety, epigastric fullness, epigastric pain and burning (the 4 cardinal symptoms pragmatically described by the Rome III Committee)[16] and other dyspeptic symptoms such as epigastric pressure, belching, nausea and vomiting was scored from 0 to 3 (0 = absent, 1 = 2 d/wk; 2 = 3-5 d/wk; and 3 = 6 d or 7 d/wk); the intensity for the same symptoms was scored from 0 to 3 (0 = absent; 1 = not very bothersome, not interfering with daily activities; 2 = bothersome, but not interfering with daily activities; and 3 = interfering with daily activities). A frequency-intensity score from 0 up to a maximum of 6 was obtained for each symptom[19].
Data are expressed as mean ± SE, unless otherwise specified. χ2 test and, analysis of variance (ANOVA) followed by one way ANOVA for multiple comparisons (Scheffè) were used to compare categorical and continuous data, respectively. The significance level was set at 0.05. The statistical program used was SPSS version 12.0 for Windows.
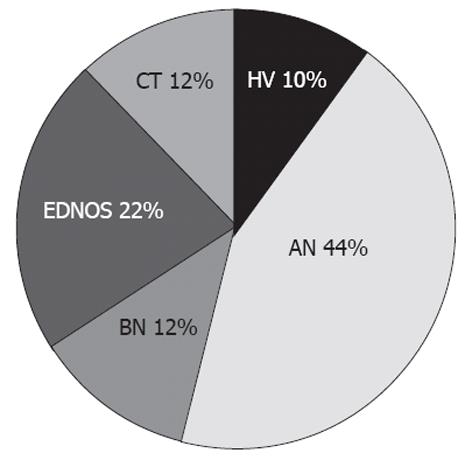
Anthropometric characteristics of the studied population were shown in Table 1. Eighteen/20 (90%) AN, 5/6 (83.3%) BN, 9/10 (90%) EDNOS, 5/9 (55.6%) CT, and 4/22 (18.2%) HV met Rome III criteria for PDS (χ2, P < 0.001). Figure 1 shows the distribution of PDS diagnosis in ED, CT and HV. Only one BN patient met the EPS Criteria. None of the patients with ED, CT, OB or HV had both PDS and EPS.
| AN (n = 20) | BN (n = 6) | EDNOS (n = 10) | CT (n = 9) | OB (n = 32) | HV (n = 22) | P value | |
| Characteristics | |||||||
| Age (yr) | 22.45 ± 0.94 | 24.83 ± 2.76 | 24.50 ± 1.82 | 24.89 ± 2.21 | 23.84 ± 0.74 | 23.67 ± 0.71 | 0.74 |
| Weight (kg) | 42.79 ± 1.18 | 60.80 ± 6.13 | 54.65 ± 2.51 | 48.13 ± 1.89 | 115.40 ± 3.27 | 60.26 ± 1.87 | < 0.001 |
| Symptom | |||||||
| Postprandial fullness | 4.15 ± 0.46 | 5.00 ± 1.00 | 4.10 ± 0.71 | 1.44 ± 0.78 | 0.00 ± 0.00 | 0.36 ± 0.17 | < 0.001 |
| Early satiety | 3.85 ± 0.50 | 1.17 ± 0.75 | 3.50 ± 0.72 | 2.11 ± 0.81 | 0.00 ± 0.00 | 0.05 ± 0.05 | < 0.001 |
| Nausea | 0.89 ± 0.38 | 3.17 ± 1.05 | 2.70 ± 0.92 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.17 ± 0.17 | < 0.001 |
| Epigastric pressure | 2.21 ± 0.55 | 4.67 ± 0.99 | 4.20 ± 0.70 | 1.22 ± 0.57 | 0.75 ± 0.23 | 0.67 ± 0.34 | < 0.001 |
| Epigastric burning | 1.05 ± 0.40 | 1.83 ± 1,17 | 1.10 ± 0.64 | 0.00 ± 0.00 | 0.44 ± 0.23 | 0.00 ± 0.00 | 0.02 |
| Epigastric pain | 1.32 ± 0.50 | 1.67 ± 0.80 | 1.80 ± 0.74 | 0.22 ± 0.22 | 0.00 ± 0.00 | 0.00 ± 0.00 | < 0.001 |
| Belching | 0.37 ± 0.23 | 1.33 ± 0.99 | 0.80 ± 0.53 | 0.78 ± 0.46 | 0.31 ± 0.20 | 0.22 ± 0.22 | 0.40 |
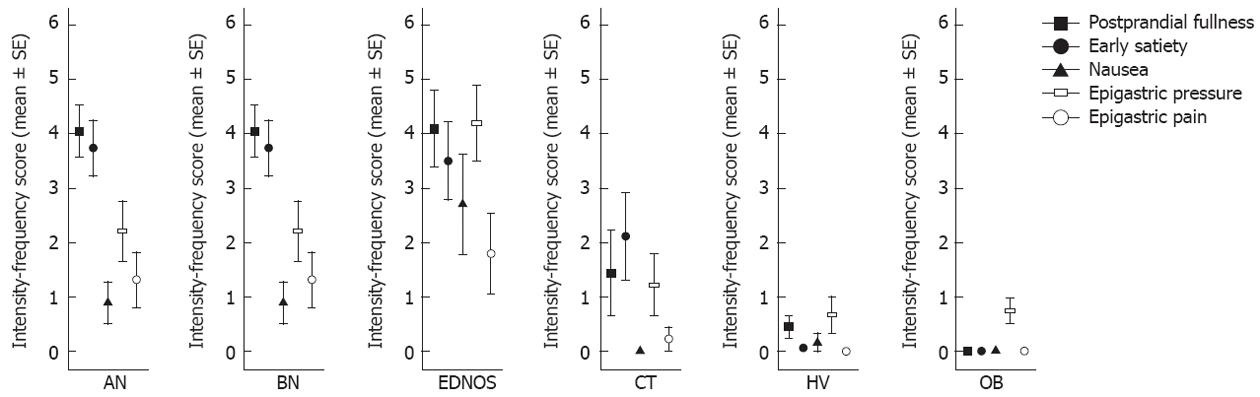
Table 1 shows the intensity-frequency score calculated for each symptom in the studied population. Postprandial fullness intensity-frequency score was significantly higher in AN, BN and EDNOS groups compared to the score calculated in the CT group (4.15 ± 2.08 vs 1.44 ± 2.35, P = 0.003; 5.00 ± 2.45 vs 1.44 ± 2.35, P = 0.003; 4.10 ± 2.23 vs 1.44 ± 2.35, P = 0.002, respectively), OB group (4.15 ± 2.08 vs 0.00 ± 0.00, P < 0.001; 5.00 ± 2.45 vs 0.00 ± 0.00, P < 0.001; 4.10 ± 2.23 vs 0.00 ± 0.00, P < 0.001, respectively) and, HV (4.15 ± 2.08 vs 0.36 ± 0.79, P < 0.001; 5.00 ± 2.45 vs 0.36 ± 0.79, P < 0.001; 4.10 ± 2.23 vs 0.36 ± 0.79, P < 0.001, respectively). Early satiety intensity-frequency score was prominent in anorectic patients compared to bulimic patients (3.85 ± 2.23 vs 1.17 ± 1.83, P = 0.015), obese patients (3.85 ± 2.23 vs 0.00 ± 0.00, P < 0.001) and, HV (3.85 ± 2.23 vs 0.05 ± 0.21, P < 0.001). Nausea and epigastric pressure were increased in bulimic and EDNOS patients. Specifically, nausea intensity-frequency score was significantly higher in BN and EDNOS patients compared to the score calculated in anorectic patients (3.17 ± 2.56 vs 0.89 ± 1.66, P = 0.04; 2.70 ± 2.91 vs 0.89 ± 1.66, P = 0.05, respectively), Constitutional Thinner subjects (3.17 ± 2.56 vs 0.00 ± 0.00, P = 0.004; 2.70 ± 2.91 vs 0.00 ± 0.00, P = 0.005, respectively), obese patients (3.17 ± 2.56 vs 0.00 ± 0.00, P < 0.001; 3.17 ± 2.56 vs 0.00 ± 0.00, P < 0.001, respectively) and, HV (3.17 ± 2.56 vs 0.17 ± 0.71, P = 0.002; 3.17 ± 2.56 vs 0.17 ± 0.71, P = 0.001, respectively). Epigastric pressure intensity-frequency score was significantly higher in bulimic and EDNOS patients compared to the score calculated in CT subjects (44.67 ± 2.42 vs 1.22 ± 1.72, P = 0.03; 4.20 ± 2.21 vs 1.22 ± 1.72, P = 0.03, respectively), obese patients (4.67 ± 2.42 vs 0.75 ± 1.32, P = 0.001; 4.20 ± 2.21 vs 0.75 ± 1.32, P < 0.001, respectively) and, HV (4.67 ± 2.42 vs 0.67 ± 1.46, P = 0.001; 4.20 ± 2.21 vs 0.67 ± 1.46, P = 0.001, respectively). Vomiting was referred in 100% of BN patients, in 20% of EDNOS patients, in 15% of AN patients, in 22% of CT subjects and, in 5.6% of HV (χ2, P < 0.001). Epigastric pain intensity-frequency score just failed to reach significance in EDNOS compared to HV (P = 0.05), whereas it was significantly higher in EDNOS compared to OB patients (P = 0.02). Figure 2 shows the pattern of dyspeptic symptoms that reached the statistical significance in all groups.
The novel result of our study was that the diagnosis of PDS according to Rome III Criteria was very common in AN, BN and EDNOS, the three main categories of ED, whilst EPS is incredibly rare. Moreover, BN and EDNOS showed high postprandial fullness, epigastric pressure and nausea intensity-frequency scores, whereas AN patients shared with BN an increase in postprandial fullness score, but conversely demonstrated a prominent early satiety. OB patients were almost asymptomatic regarding FD symptoms.
The hallmarks of ED are clinical disturbances in body image and eating behavior resulting in physical and psychological impairment. These clinical entities are diagnosed according to DSM-IV criteria Among them disorders such as AN, BN and EDNOS are more common in women and can result in long-term health consequences even in increased mortality. The core presentation of Anorexia nervosa is characterized by the inability or refusal to maintain a minimally normal weight, a profoundly distorted perception of body weight and shape, and amenorrhea. Under the definition of BN are included individuals who engage in recurrent binge-eating episodes and recurrent inappropriate compensatory behaviours that are intended to rid calories that they voraciously ingested. EDNOS involves milder versions of anorexia and bulimia nervosa that do not satisfy all the criteria. Previous studies have suggested that anorectic patients frequently complain of gastrointestinal symptoms hinting at a disordered gastric motility, especially when they are in a refeeding phase[20]. Dyspeptic symptoms such as epigastric fullness and distension were found to be significantly more prevalent and intense than in healthy subjects[21-23] and may serve as an argument for food refusal[24]. However, they are often overlooked or misinterpreted. In this study the more prevalent and intense dyspeptic symptoms scored by a standardized questionnaire were epigastric fullness and early satiety. In bulimic patients the large quantities eaten during a binge not only lead to a feeling of loss of control but also to a sensation of epigastric distension. The latter as well as the often associated epigastric pain are terminated by self-induced vomiting, which allows either continuation or termination of the binge[20]. Our findings demonstrated that BN and EDNOS referred postprandial fullness, epigastric pressure and nausea as their most prevalent and intense dyspeptic symptoms. The mechanisms underlying these dyspeptic symptoms in ED are still unclear, although malnutrition and the resultant metabolic myopathy, along with electrolyte depletion seem to play the crucial rolein determining the demonstrated abnormalities in gastric empting[22], gastric capacity[25] and, blunted endocrine control[26]. Conversely, irrespective of the pathophysiology and mechanisms involved, it is intriguing that the association of higher body mass index alone with dyspeptic symptoms was relatively modest also contrary to the study expectation. It is noteworthy that in our OB group no binge behavior has been diagnosed, suggesting that eating patterns are more closely linked to symptom generation in the GI tract[27]. In addition, to our knowledge this is the first study that demonstrated in ED a high prevalence of PDS using the Rome III criteria, an international accepted instrument. Another novel finding of this study was that 55% of CT subjects met the Rome III criteria for PDS and referred a higher intensity-frequency score for early satiety than healthy volunteers. Individuals with CT belong to a non pathological state, poorly described[28]. They are often young women, severely thin that continue to have a close to normal fat mass percentage, normal physiological menstrual cycles, no detectable abnormalities of cortisol, insulin-like growth factor 1, or free T3 secretory patterns and normal energy metabolism[17,28]. The mechanism behind low-weight steadiness in CT was not yet elucidated. Multifactorial etiology involves a combination of genetics in addition to as yet unrecognized pathophysiological factors[29]. CT subjects display an equilibrated energy metabolism similar to that of control subjects. CT subjects attempt to gain weight, often overeating. To assess whether this eating pattern is related to GI symptom generation, further dynamic studies are needed.
Our findings leave room for speculation on the mechanisms underlying FD in patients with an ED. It has been suggested that FD results from a closed interaction of biological, psychosocial and social factors[30]. The altered eating behavior seen in EDs is strongly associated with disturbed gastrointestinal sensitivity and motor physiology[8]. ED and FD patients shared a high prevalence of psychiatric comorbidities[31]. These latter together with the motor and sensitivity disturbances can lay the foundation of an FGID. Once established the psychological and physiological disturbances can perpetuate and strengthen each other resulting in an FGID that can persist independently of the ED that originally caused the motor and sensitivity disturbances[7]
It is also conceivable that a large number of individuals presenting for medical treatment for GI symptoms in gastroenterologic outpatient clinics could be better managed by firstly the identification and, secondly by receiving adequate treatment for concurrent ED. This is an important issue given that the ultimate goal of therapy in suspected ED patients is the normalization of gastric motor function with the resumption of normal eating behavior enabling the patient’s social reintegration and restoration to an appearance acceptable to the social environment.
We acknowledge the limitations of this study. Firstly, the overall sample size was small. Furthermore, the study was limited by the failure to screen for organic GI disorder which, although quite rare in patients with EDs[32], could falsely inflate estimates of FD incidence.
In conclusion, the high prevalence of meal-related symptoms in ED patients should encourage in gastroenterology outpatient clinics the routine screening for ED. In addition to perhaps helping design more efficacious interventions for FD if patterns of food ingestion contribute to the development of unexplained GI symptoms, further studies are necessary to demonstrate whether patterns of food ingestion contribute to the development of unexplained GI symptoms. This attention to eating patterns might provide a simple, safe and potentially effective method to better manage FD patients too.
Eating disorders (ED) are highly prevalent health problems in Western countries, especially in young women. Three main ED categories have been identified on the basis of the diagnostic and statistical manual of mental disorders, 4th edition: anorexia nervosa (AN), bulimia nervosa (BN), and eating disorders not otherwise specified (EDNOS). Gastrointestinal (GI) symptoms are a common complaint in ED patients. It is a common occurrence that patients, before presenting to healthcare services with an ED, seek treatment for GI symptoms.
A previous study demonstrated that 98% of ED patients fulfilled the criteria for at least one functional gastrointestinal disorder (FGIDs) such as irritable bowel syndrome, functional heartburn, functional abdominal bloating, functional constipation, functional dysphagia and functional anorectal pain disorder. Recently, a high prevalence of irritable bowel symptoms was confirmed in patients already affected by ED. However, it was suggested that FGIDs were the result of the behaviour-associated ED and that, these GI symptoms may persist even after the recovery from ED, especially in psychologically distressed patients. Currently, the underlying mechanisms that link ED and GI symptoms remain to be elucidated.
The novel result of the study was that AN, BN and EDNOS, the three main categories of ED, had a high prevalence of dyspeptic symptoms fulfilling the Rome III criteria to positively diagnose postprandial distress syndrome (PDS), not epigastric pain syndrome (EPS). Moreover, BN and EDNOS showed high postprandial fullness, epigastric pressure and nausea intensity-frequency scores, whereas AN patients shared with BN an increase in postprandial fullness score, but conversely demonstrated a prominent early satiety. Irrespective of the pathophysiology and mechanisms involved, it is intriguing that the association of higher body mass index alone with dyspeptic symptoms was relatively modest, also contrary to the study expectations. In addition, to the knowledge, this is the first study that demonstrated in ED a high prevalence of PDS using the Rome III criteria, an internationally accepted instrument. Another interesting finding of the study was that 55% of constitutional thinness subjects (CT) met the Rome III criteria for PDS and, referred a higher intensity-frequency score for early satiety than healthy volunteers.
It is conceivable that a large number of individuals presenting for medical treatment for GI symptoms in gastroenterologic outpatient clinics could be better managed by the identification of a concurrent ED. Their findings leave room for speculation on the mechanisms underlying functional dyspepsia (FD) in patients with an ED. The altered eating behavior seen in EDs is strongly associated with impairment in gastrointestinal sensitivity and motor physiology. ED and FD patients shared a high prevalence of psychiatric comorbidities. These latter together with the motor and sensitivity disturbances can lay the foundation of an FGID. Once established the psychological and physiological disturbances can perpetuate and strengthen each other resulting in an FGID that can persist independently of the ED that originally caused the motor and sensitivity disturbances. Further studies are needed in the future to demonstrate these hypotheses.
ED are clinical disturbances in body image and eating behavior resulting in physical and psychological impairment. These clinical entities are diagnosed according to diagnostic and statistical manual of mental disorders, 4th edition criteria; AN is characterized by the inability or refusal to maintain a minimally normal weight, a profoundly distorted perception of body weight and shape, and amenorrhea; BN is a clinical entity that includes individuals who engage in recurrent binge-eating episodes and recurrent inappropriate compensatory behaviours that are intended to rid calories that they voraciously ingested; EDNOS involves milder versions of anorexia and bulimia nervosa that do not satisfy all the criteria; CT is a non pathological state, poorly described. Subjects constitutionally thin are often young women, severely thin that continue to have a close to normal fat mass percentage and, normal physiological menstrual cycles; Rome III riteria to diagnose FD are defined as the presence of symptoms thought to originate in the gastroduodenal region, in the absence of any organic, systemic, or metabolic disease that is likely to explain the symptoms. The specific symptoms needed to diagnose FD are: epigastric pain, epigastric burning, post-prandial fullness and early satiation; PDS is characterized by bothersome postprandial fullness or early satiation; EPS is characterized by bothersome unexplained epigastric pain or burning.
This is a good descriptive study. The results are interesting and suggest that due to the high prevalence of dyspepsia symptoms in patients already diagnosed for ED, it could be recommended to gastroenterologists to evaluate patients seeking treatment for the post-prandial distress syndrome to rule out a possible coexistence of any ED.
Peer reviewers: Frank I Tovey, OBE, ChM, FRCS, Honorary Research Felllow, Department of Surgery, University College London, London W1W 7EJ, United Kingdom; Cesare Tosetti, MD, Department of Primary Care, Health Care Agency of Bologna, Via Rosselli 21, 40046 Porretta Terme, Italy
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. J Abnorm Psychol. 1993;102:133-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1075] [Cited by in F6Publishing: 947] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 2. | Fairburn CG, Harrison PJ. Eating disorders. Lancet. 2003;361:407-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1101] [Cited by in F6Publishing: 902] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 3. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association 2000; . [Cited in This Article: ] |
| 4. | Bohn K, Doll HA, Cooper Z, O'Connor M, Palmer RL, Fairburn CG. The measurement of impairment due to eating disorder psychopathology. Behav Res Ther. 2008;46:1105-1110. [PubMed] [Cited in This Article: ] |
| 5. | Boyd C, Abraham S, Kellow J. Psychological features are important predictors of functional gastrointestinal disorders in patients with eating disorders. Scand J Gastroenterol. 2005;40:929-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Dejong H, Perkins S, Grover M, Schmidt U. The prevalence of irritable bowel syndrome in outpatients with bulimia nervosa. Int J Eat Disord. 2011;44:661-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Porcelli P, Leandro G, De Carne M. Functional gastrointestinal disorders and eating disorders. Relevance of the association in clinical management. Scand J Gastroenterol. 1998;33:577-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Janssen P. Can eating disorders cause functional gastrointestinal disorders? Neurogastroenterol Motil. 2010;22:1267-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ogg EC, Millar HR, Pusztai EE, Thom AS. General practice consultation patterns preceding diagnosis of eating disorders. Int J Eat Disord. 1997;22:89-93. [PubMed] [Cited in This Article: ] |
| 10. | Horwitz BJ, Fisher RS. The irritable bowel syndrome. N Engl J Med. 2001;344:1846-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 153] [Article Influence: 6.7] [Reference Citation Analysis (2)] |
| 11. | Camilleri M, Dubois D, Coulie B, Jones M, Kahrilas PJ, Rentz AM, Sonnenberg A, Stanghellini V, Stewart WF, Tack J. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 213] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1502] [Cited by in F6Publishing: 1393] [Article Influence: 44.9] [Reference Citation Analysis (1)] |
| 14. | Jones RH, Lydeard SE, Hobbs FD, Kenkre JE, Williams EI, Jones SJ, Repper JA, Caldow JL, Dunwoodie WM, Bottomley JM. Dyspepsia in England and Scotland. Gut. 1990;31:401-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 15. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1467] [Cited by in F6Publishing: 1420] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 16. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1243] [Cited by in F6Publishing: 1154] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 17. | Bossu C, Galusca B, Normand S, Germain N, Collet P, Frere D, Lang F, Laville M, Estour B. Energy expenditure adjusted for body composition differentiates constitutional thinness from both normal subjects and anorexia nervosa. Am J Physiol Endocrinol Metab. 2007;292:E132-E137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Formiguera X, Cantón A. Obesity: epidemiology and clinical aspects. Best Pract Res Clin Gastroenterol. 2004;18:1125-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Amato G, Limongelli P, Pascariello A, Rossetti G, Del Genio G, Del Genio A, Iovino P. Association between persistent symptoms and long-term quality of life after laparoscopic total fundoplication. Am J Surg. 2008;196:582-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Stacher G. Gut function in anorexia nervosa and bulimia nervosa. Scand J Gastroenterol. 2003;38:573-587. [PubMed] [Cited in This Article: ] |
| 21. | Herpertz-Dahlmann BM, Wewetzer C, Schulz E, Remschmidt H. Course and outcome in adolescent anorexia nervosa. Int J Eat Disord. 1996;19:335-345. [PubMed] [Cited in This Article: ] |
| 22. | Stacher G, Kiss A, Wiesnagrotzki S, Bergmann H, Höbart J, Schneider C. Oesophageal and gastric motility disorders in patients categorised as having primary anorexia nervosa. Gut. 1986;27:1120-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Robinson PH, Clarke M, Barrett J. Determinants of delayed gastric emptying in anorexia nervosa and bulimia nervosa. Gut. 1988;29:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Lee S, Lee AM, Ngai E, Lee DT, Wing YK. Rationales for Food Refusal in Chinese Patients with Anorexia Nervosa. Int J Eat Disord. 2001;29:224-229. [PubMed] [Cited in This Article: ] |
| 25. | Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. 1992;56:656-661. [PubMed] [Cited in This Article: ] |
| 26. | Devlin MJ, Walsh BT, Guss JL, Kissileff HR, Liddle RA, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr. 1997;65:114-120. [PubMed] [Cited in This Article: ] |
| 27. | Cremonini F, Camilleri M, Clark MM, Beebe TJ, Locke GR, Zinsmeister AR, Herrick LM, Talley NJ. Associations among binge eating behavior patterns and gastrointestinal symptoms: a population-based study. Int J Obes (Lond). 2009;33:342-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, Dardennes R, Mounier C, Zizzari P, Lang F. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. 2003;88:109-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Bulik CM, Allison DB. The genetic epidemiology of thinness. Obes Rev. 2001;2:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Oustamanolakis P, Tack J. Dyspepsia: organic versus functional. J Clin Gastroenterol. 2012;46:175-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45 Suppl 2:II25-II30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Kiss A, Wiesnagrotzki S, Abatzi TA, Meryn S, Haubenstock A, Base W. Upper gastrointestinal endoscopy findings in patients with long-standing bulimia nervosa. Gastrointest Endosc. 1989;35:516-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |