Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.7033
Revised: November 19, 2012
Accepted: November 24, 2012
Published online: December 21, 2012
AIM: To investigate the impact of renal and graft function on post-transplant hyperlipidemia (PTHL) in living donor liver transplantation (LDLT).
METHODS: A total of 115 adult patients undergoing LDLT from January 2007 to May 2009 at a single center were enrolled. Data were collected and analyzed by the China Liver Transplant Registry retrospectively. PTHL was defined as serum triglycerides ≥ 150 mg/dL or serum cholesterol ≥ 200 mg/dL or the need for pharmacologic treatment at the sixth month after LDLT. Early renal dysfunction (ERD) was defined as serum creatinine ≥ 2 mg/dL and/or the need for renal replacement therapy in the first post-transplant week.
RESULTS: In 115 eligible patients, the incidence of PTHL was 24.3%. Recipients with PTHL showed a higher incidence of post-transplant cardiovascular events compared to those without PTHL (17.9% vs 4.6%, P = 0.037). Serum creatinine showed significant positive correlations with total serum triglycerides, both at post-transplant month 1 and 3 (P < 0.01). Patients with ERD had much higher pre-transplant serum creatinine levels (P < 0.001) and longer duration of pre-transplant renal insufficiency (P < 0.001) than those without ERD. Pre-transplant serum creatinine, graft-to-recipient weight ratio, graft volume/standard liver volume ratio, body mass index (BMI) and ERD were identified as risk factors for PTHL by univariate analysis. Furthermore, ERD [odds ratio (OR) = 9.593, P < 0.001] and BMI (OR = 6.358, P = 0.002) were identified as independent risk factors for PTHL by multivariate analysis.
CONCLUSION: Renal function is closely associated with the development of PTHL in LDLT. Post-transplant renal dysfunction, which mainly results from pre-transplant renal insufficiency, contributes to PTHL.
- Citation: Ling Q, Wang K, Lu D, Guo HJ, Jiang WS, He XX, Xu X, Zheng SS. Major influence of renal function on hyperlipidemia after living donor liver transplantation. World J Gastroenterol 2012; 18(47): 7033-7039
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/7033.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.7033
Post-transplant hyperlipidemia (PTHL) is a common and serious complication in liver transplantation (LT). It is estimated that 45%-70% of LT recipients develop PTHL[1-4]. Previous studies have shown that recipients with lipid metabolic disorders are approximately 3-4 times more likely to have cardiovascular events than recipients without lipid metabolic disorders[5,6]. Therefore, PTHL is well recognized as one of the major risk factors for cardiovascular diseases, including myocardial infarction, ischemic stroke and peripheral arterial disease. Furthermore, cardiovascular complications after LT exert negative effects on the recipient’s long-term survival and quality of life and are becoming the leading cause of non-graft related deaths in LT recipients[7-9].
The pathogenesis of PTHL remains to be elucidated. Possible factors that may increase the risk of PTHL include overweight or obesity, advanced age, and the use of immunosuppressive agents[2,10]. Immunosuppressive drugs were considered to have a major effect on the development of metabolic syndromes in previous years. Today, immunosuppressive drugs are known to have a reduced impact on glucose and lipid metabolism[11]. Low toxicity immunosuppressive protocols (e.g., early withdrawal of steroids and low-dose calcineurin inhibitors) have decreased the impact of immunosuppressive drugs on PTHL. It is known that the liver plays an essential role in lipoprotein metabolism and is involved in almost every key step during lipid anabolism and catabolism. In living donor liver transplantation (LDLT), graft function can be influenced by reduced graft size, preservation and ischemia-reperfusion injuries. Early allograft dysfunction (EAD) may occur in more than 10% of LT recipients and result in an unfavorable prognosis[12,13]. More importantly, renal insufficiency, which is common in liver transplant candidates, is closely associated with significant alterations in lipid metabolism. Early renal dysfunction (ERD), which is also common after LT, may indicate a prolonged recovery of kidney insufficiency and indicates a poor outcome[14].
To date, it is still unknown whether impaired kidney or graft function can cause derangements in lipid metabolism after LDLT. To clarify these questions, we designed this retrospective study with the aim of evaluating the impact of renal and graft function on lipid metabolism after LDLT and to identify the possible risk factors for PTHL.
All adult (age ≥ 18 years old) patients undergoing LDLT from January 2007 to May 2009 at the First Affiliated Hospital, Zhejiang University School of Medicine, China were included in this retrospective study. Patients who died within 3 mo of LDLT were excluded. A total of 115 (96 male and 19 female) patients with a mean age of 44.7 ± 10.11 years (median: 46 years, range: 18-64 years) at transplantation were finally included. The indications for LT included cirrhosis (n = 63, 54.8%), acute liver failure (n = 28, 24.3%) and hepatocellular carcinoma (n = 24, 20.9%). Patient characteristics are shown in Table 1. Written informed consent was acquired from all donors and recipients before transplantation. Each organ donation and transplantation at our center was strictly carried out under the guidelines of the Ethics Committee of the First Affiliated Hospital, Zhejiang University, the regulation of Organ Transplant Committee of Zhejiang Province and the Helsinki Declaration of 1975. No prisoners were included in this study.
| PTHL group(n = 28) | Non-PTHL group (n = 87) | P value | |
| Donor | |||
| Age (yr) | 24.8 ± 4.3 | 24.0 ± 6.4 | NS |
| Male/female (n) | 27/1 | 80/7 | NS |
| Cold ischemia time (h) | 1.2 ± 0.5 | 1.1 ± 0.4 | NS |
| GR/WR (%) | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.048 |
| GV/SLV (%) | 58.5 ± 5.6 | 62.6 ± 10.7 | 0.011 |
| Hepatic steatosis n (%) | 2 (7.1) | 7 (8.0) | NS |
| Serum triglyceride (mg/dL) | 90.9 ± 42.4 | 96.2 ± 45.0 | NS |
| Serum cholesterol (mg/dL) | 137.3 ± 30.9 | 135.7 ± 27.1 | NS |
| Recipient | |||
| Age (yr) | 45.4 ± 8.2 | 44.5 ± 10.6 | NS |
| Male/female (n) | 27/1 | 69/18 | NS |
| Primary liver diseases n (%) | |||
| Cirrhosis | 12 (42.9) | 51 (58.6) | NS |
| Acute liver failure | 9 (32.1) | 19 (21.8) | NS |
| Hepatocellular carcinoma | 7 (25) | 17 (19.5) | NS |
| Pre-transplant metabolic status | |||
| BMI (kg/m2) | 23.2 ± 3.16 | 21.9 ± 2.79 | 0.036 |
| Smoking n (%) | 7 (25) | 19 (21.8) | NS |
| Alcohol abuse n (%) | 3 (10.7) | 13 (14.9) | NS |
| Diabetes mellitus n (%) | 2 (7.1) | 4 (4.6) | NS |
| Hypertension n (%) | 3 (10.7) | 5 (5.7) | NS |
| Hyperlipidemia n (%) | 4 (14.3) | 15 (17.2) | NS |
| Pre-transplant MELD score | 23.8 ± 12.4 | 20.1 ± 9.6 | NS |
| Total bilirubin (mg/dL) | 14.2 (1.0-36.5) | 12.8 (0.8-33.6) | NS |
| International normalized ratio | 1.6 ± 1.1 | 1.4 ± 0.6 | NS |
| Serum creatinine (mg/dL) | 0.9 (0.5-4.0) | 0.7 (0.4-4.5) | 0.004 |
| Post-transplant tacrolimus level (ng/mL) | |||
| Month 1 | 8.1 ± 2.8 | 9.8 ± 4.6 | NS |
| Month 3 | 9.2 ± 2.6 | 8.3 ± 2.6 | NS |
| Month 6 | 6.7 ± 2.2 | 7.7 ± 2.7 | NS |
| Follow-up period (yr) | 2.7 ± 1.2 | 2.6 ± 0.9 | NS |
All patients received a triple immunosuppressive regimen incorporating tacrolimus, prednisolone, and mycophenolate mofetil as described previously[15]. An interleukin (IL)-2 receptor blocker was used in some patients. Prednisolone was withdrawn within the first post-transplant month. A reduced dose of tacrolimus was given to patients who developed post-transplant renal impairment. All patients were routinely followed up at the out-patient clinic and the mean follow-up time was 2.66 ± 1.02 years (median: 1.98 years, range: 0.50-4.63 years).
The data were extracted from the China Liver Transplant Registry (CLTR) database: age (donor/recipient), gender (donor/recipient), underlying liver diseases, cold ischemic time, graft-to-recipient weigh ratio (GR/WR), graft volume to standard liver volume ratio (GV/SLV), donor’s hepatic steatosis, recipient’s pre-transplant metabolic status [history of smoking, alcohol abuse, diabetes mellitus, hypertension, hyperlipidemia, body mass index (BMI)], recipient’s post-transplant liver function (bilirubin, alanine transaminase, aspartate transaminase, cholinesterase and international normalized ratio), recipient’s pre/post-transplant renal function (serum creatinine), donor’s and recipient’s pre/post-transplant serum lipid profile (total triglycerides, total cholesterol), immunosuppressive regimen (agents and plasma levels), and recipient’s post-transplant complications.
Hyperlipidemia was defined as serum triglycerides ≥ 150 mg/dL or serum cholesterol ≥ 200 mg/dL or the need for pharmacologic treatment[16]. Patients were divided into the PTHL group (n = 28) and non-PTHL group (n = 87) according to their serum lipid levels at the sixth month after LDLT. EAD was defined by the presence of at least one of the following features: total bilirubin > 10 mg/dL, prothrombin time ≥ 17 s, or hepatic encephalopathy from day 2 to 7 post-transplantation[17]. ERD was defined as serum creatinine ≥ 2 mg/dL and/or the need for renal replacement therapy in the first post-transplant week[18]. Renal insufficiency was defined as pre-transplant serum creatinine > 1.5 mg/dL[18].
Quantitative variables were presented as mean ± SD or median with range. Categorical variables were expressed as numbers and percentages. The Student’s t test or Mann-Whitney U test was used to compare quantitative variables and the χ2 test was used to compare categorical variables. Pearson’s correlation and scatter plot graph were used for correlation analysis. Logistic regression was used to identify the risk factors for PTHL. Variables that were statistically significant in univariate analysis were entered into multivariate analysis. The Kaplan-Meier method and log-rank test were used for survival analysis. SAS software version 9.2 (SAS institute, Cary, NC, United States) was used to complete all the analyses, and a P value of less than 0.05 was considered statistically significant. Data analysis was performed by the CLTR Statistical Department.
Of 115 eligible recipients, 19 (16.5%) had pre-transplant hyperlipidemia and 28 (24.3%) developed PTHL. Of the recipients with and without pre-transplant hyperlipidemia, the incidence of PTHL did not differ significantly (21.1% vs 25%, P = 0.714).
Of 115 donors, 13 (11.3%) were diagnosed with hyperlipidemia and 9 (7.8%) had hepatic steatosis. However, neither donor hyperlipidemia nor hepatic steatosis was found to be differently distributed between the PTHL group and non-PTHL group (both P > 0.05) (Table 1).
Nine recipients (7.8%) developed cardiovascular events, including four with nonfatal myocardial infarction, two with cardiac arrhythmia, one with cardiac arrest and two with stroke, with a mean onset time of 10.5 mo (median: 8 mo, range: 3-15 mo). Compared to the non-PTHL group, the PTHL group had a higher incidence of cardiovascular events after LDLT (n = 5, 17.9% vs n = 4, 4.6%, P = 0.037). The 1- and 3-year cumulative survival rates were 89.3% and 85.7% in the PTHL group, and 97.7% and 85.0% in the non-PTHL group, respectively. There was no statistically significant difference between the two groups in cumulative survival (P = 0.956).
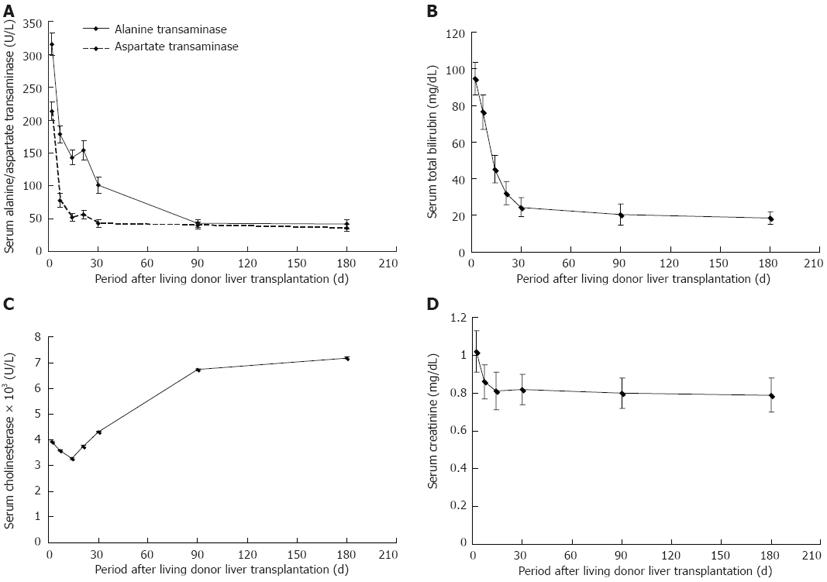
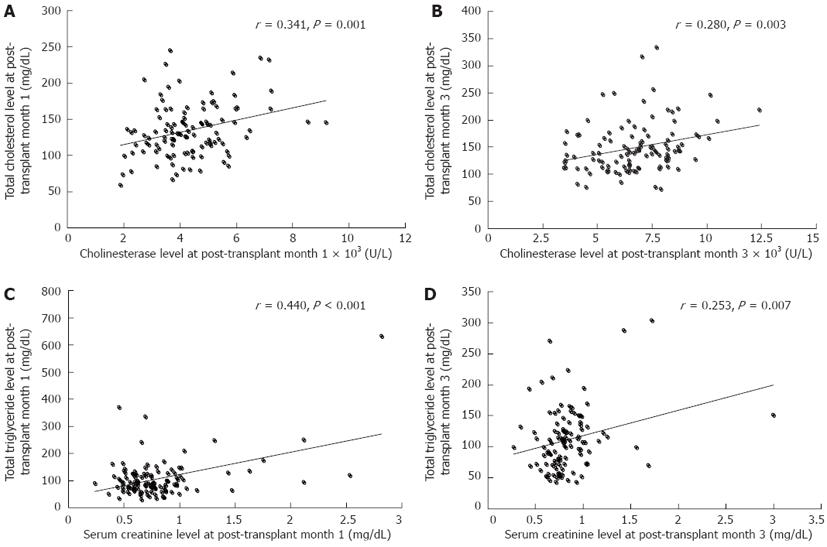
As shown in Figure 1, alanine transaminase, aspartate transaminase and bilirubin, which indicate hepatic cellular injury, decreased sharply in the first post-transplant month and continued to decline to the normal range during the next two months. Cholinesterase, which represents hepatocellular synthetic function, remained at a low level during the first post-transplant month and gradually increased to an ideal level at post-transplant month 3. Of these parameters, cholinesterase had a significant positive correlation with serum cholesterol level at post-transplant month 1 (r = 0.341, P = 0.001) and month 3 (r = 0.280, P = 0.003), respectively (Figure 2A and B). However, cholinesterase was not associated with the development of PTHL following logistic regression analysis (P > 0.05).
EAD occurred in 18 (15.7%) of 115 recipients. At the third post-transplant month, EAD had resolved in 77.8% (14/18) of patients. Primary graft non-function was not observed. The incidence of PTHL did not differ significantly between patients with EAD and those without EAD (33.3% vs 20.6%, P = 0.236).
Serum creatinine levels decreased during the first post-transplant month (Figure 1) and were significantly related to serum triglyceride level at the first (r = 0.440, P < 0.001) and third (r = 0.250, P = 0.007) post-transplant month, respectively (Figure 2C and D). Serum creatinine levels at the first post-transplant month differed significantly between the PTHL and non-PTHL groups (1.10 ± 0.67 mg/dL vs 0.73 ± 0.25 mg/dL, P = 0.001).
ERD occurred in 16 (13.9%) of 115 post-transplant recipients. There was a statistically significant difference between ERD and non-ERD recipients in pre-transplant renal function (Table 2). Serum creatinine remained high during the first 6 post-transplant months in ERD recipients. Of the 16 recipients with ERD, 9 and 5 needed hemodialysis in the first and third post-transplant month, respectively. The prevalence of PTHL in ERD patients was significantly higher than that in non-ERD patients (56.3% vs 19.2%, P = 0.003), notably for hypertriglyceridemia (56.3% vs 12.1%, P < 0.001).
| ERD(n = 16) | Non-ERD(n = 99) | P value | |
| Pre-transplant | |||
| MELD | 30.1 ± 11.7 | 19.5 ± 9.4 | < 0.001 |
| Total bilirubin (mg/dL) | 15.2 (1.8-29.8) | 13.6 (0.8-36.5) | NS |
| International normalized ratio | 1.6 ± 0.9 | 1.4 ± 0.7 | NS |
| Serum creatinine (mg/dL) | 2.2 (0.7-4.0) | 0.8 (0.5-4.5) | < 0.001 |
| Renal insufficiency > 15 d n (%) | 11 (68.8) | 15 (15.2) | < 0.001 |
| Hemodialysis n (%) | 11 (68.8) | 5 (5.1) | < 0.001 |
| Serum triglyceride (mg/dL) | 77.7 ± 58.4 | 77.7 ± 62.7 | NS |
| Serum cholesterol (mg/dL) | 92.7 ± 42.9 | 119.3 ± 55.6 | NS |
| Post-transplant | |||
| Serum creatinine (mg/dL) | |||
| At month 1 | 1.4 (0.6-2.8) | 0.7 (0.2-1.2) | < 0.001 |
| At month 3 | 1.0 (0.6-3.0) | 0.7 (0.3-1.3) | 0.008 |
| At month 6 | 1.1 (0.6-4.1) | 0.7 (0.3-1.5) | < 0.001 |
| Total bilirubin (mg/dL) | |||
| At month 1 | 1.3 (0.4-4.3) | 1.1 (0.3-5.8) | NS |
| At month 3 | 0.6 (0.2-2.5) | 0.7 (0.3-2.5) | NS |
| At month 6 | 0.6 (0.3-2.0) | 0.8 (0.4-2.8) | NS |
| Cholinesterase (U/L) | |||
| At month 1 | 3681.3 ± 1354.3 | 4403.9 ± 1323.6 | NS |
| At month 3 | 5996.7 ± 1619.4 | 6830.7 ± 1750.5 | NS |
| At month 6 | 7255.4 ± 1662.9 | 7171.5 ± 1721.6 | NS |
| Hypertriglyceridemia n (%) | 9 (56.3) | 12 (12.1) | < 0.001 |
| Hypercholesterolemia n (%) | 3 (18.8) | 9 (9.1) | NS |
| PTHL n (%) | 9 (56.3) | 19 (19.2) | 0.003 |
We postulated that donors’ pre-transplant hyperlipidemia and hepatic steatosis, GR/WR (%) and GV/SLV (%); recipients’ pre-transplant serum creatinine level, pre-transplant BMI and pre-transplant hyperlipidemia, post-transplant tacrolimus level, post-transplant diabetes within the first six months after LDLT[15], post-transplant BMI at the sixth month after LDLT, EAD and ERD were potential risk factors for PTHL. Following univariate analysis, five variables were identified as significant risk factors for PTHL and were entered into the multivariate analysis (Table 3). We subsequently found that ERD [odds ratio (OR) = 9.593, P < 0.001] and BMI (OR = 6.358, P = 0.002) were independent risk factors for PTHL.
| Univariate analysis | Multivariate analysis | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Pre-transplant serum creatinine | ≥ 1.5 mg/dL | 3.810 (1.203-12.063) | 0.023 | NS | |
| < 1.5 mg/dL | |||||
| GR/WR (%) | < 1.0 | 2.667 (1.116-6.372) | 0.027 | NS | |
| ≥ 1.0 | |||||
| GV/SLV (%) | < 60 | 2.806 (1.158-6.798) | 0.022 | NS | |
| ≥ 60 | |||||
| Pre-transplant BMI | ≥ 25 kg/m2 | 4.105 (1.435-11.752) | 0.008 | 6.358 (2.026-19.958) | 0.002 |
| < 25 kg/m2 | |||||
| ERD | With | 5.143 (1.789-16.383) | 0.003 | 9.593 (2.803-32.827) | < 0.001 |
| Without | |||||
The most important finding in this study was the correlation between renal function and PTHL, which may be of great help in better understanding the pathogenesis of PTHL, and as much as possible, preventing its development. It was demonstrated that post-transplant serum creatinine had a significant positive correlation with serum triglycerides both in the first and third post-transplant month. More importantly, using multivariate regression analysis, ERD was found to be an independent risk factor for PTHL. Patients with ERD usually had a relatively long-term and severe renal insufficiency before transplantation, and impaired renal function resolved very slowly during the first six months after transplantation. Here, we provide the first evidence that delayed recovery of renal function after transplantation, which was probably due to chronic kidney injury before transplantation, can result in PTHL, especially hypertriglyceridemia.
In fact, it is well known that chronic kidney disease is associated with hyperlipidemia. In the kidney, the apical surface of proximal tubules has a high capacity for receptor-mediated uptake of filtered lipid-binding plasma proteins[19]. Therefore, hyperlipidemia induced by impaired renal function is characterized by abnormal metabolism of plasma lipoproteins[20,21]. The reduced catabolic rate of triglyceride-rich lipoproteins and the growing hepatic production of triglyceride-high lipoproteins may explain why hypertriglyceridemia is one of the most common quantitative lipid abnormalities in patients with chronic kidney disease[22,23]. Consistent with previous findings, the impaired renal function and recovered liver synthetic function in patients with ERD during the early post-transplant period may explain the development of hypertriglyceridemia in this study. Therefore, more emphasis should be placed on this issue, as more and more patients are undergoing liver transplantation with higher serum creatinine levels in the era of MELD.
Another finding in this study was that graft function was associated with cholesterol metabolism after LDLT. Our results showed the smooth recovery of decompensated liver function during the early post-transplant period. Furthermore, elevated serum cholinesterase level, which represents the recovery of hepatic cellular synthetic function, was significantly correlated with an increase in cholesterol level within the normal range. It is noteworthy that cholesterol plays an active role during liver regeneration. It is not only a structural component, but also a significant regulator in the control of the intermediate metabolism of different liver cell types[24]. This implies that there may be mutual benefits between improved graft synthetic function and cholesterol homeostasis.
Since donor pre-transplant serum lipid level may play an essential role in the development of PTHL[25], we also analyzed the association between pre-transplant donor serum lipid level and post-transplant recipient serum lipid level. No significant difference in the incidence of PTHL was found between the recipients receiving grafts from pre-transplant hyperlipidemic and non-hyperlipidemic donors. The pre-transplant serum triglyceride and cholesterol levels of donors in the PTHL group were not significantly higher than those in the non-PTHL group. Therefore, in this study, there was no obvious evidence to show that pre-transplant donors’ serum lipid level or fatty liver exerted crucial effects on the development of PTHL.
High BMI was found to be another independent risk factor for PTHL in this study. As a main component of the metabolic syndrome, high BMI or obesity before LT has been reported to be associated with an increased prevalence of PTHL[26]. It seems unlikely that dietary habits and unhealthy lifestyle can be changed and thus patients retain their obesity status, resulting in abnormalities in lipid metabolism. It was notable that some patients had pre-transplant hyperlipidemia, but did not have PTHL in this study. A possible reason for this is that pre-transplant hyperlipidemia, which resulted mainly from hepatorenal syndrome due to end-stage liver disease, could be improved or resolved by the recovery of liver and kidney function after liver transplantation.
A high blood concentration of tacrolimus has been reported to contribute to PTHL in LDLT[27]. However, in the present study, we did not find an association between immunosuppressive drugs and PTHL. This may be due to the early steroid withdrawn and low tacrolimus concentration protocol. Furthermore, reduced-dose tacrolimus with or without an IL-2 receptor blocker was given to patients who developed post-transplant renal impairment, which also minimizes the side-effects of immunosuppressive drugs on lipid metabolism.
There were some limitations in this study. Firstly, it was not a prospective study. The impact of graft and kidney function on the development of PTHL requires confirmation in prospective studies with larger samples. Secondly, a longer period of follow-up is necessary to identify the natural history of PTHL and to determine the influence of PTHL on a recipient’s prognosis. Thirdly, a study at the molecular level should be performed as the donor’s genotype may play a role in the development of metabolic diseases.
In conclusion, renal and graft function correlated with lipid metabolism after LDLT. Severe pre-transplant renal insufficiency may lead to long-term post-transplant renal dysfunction, and consequently cause PTHL, especially hypertriglyceridemia. Appropriate clinical treatment such as the prophylactic use of fibrates or statins may be considered to prevent PTHL in patients who develop ERD or have longstanding pre-transplant renal dysfunction. Well-designed and large-sample studies are needed to verify these results.
Post-transplant hyperlipidemia (PTHL) is a major and serious complication after liver transplantation. It is estimated that 45%-70% of recipients develop PTHL, which is well recognized as one of the major risk factors for cardiovascular diseases, including myocardial infarction, ischemic stroke and peripheral arterial disease, and exerts negative effects on the recipient’s long-term survival and quality of life. It is well known that the liver plays an essential role in lipoprotein metabolism and is involved in almost every key step during lipid anabolism and catabolism. More importantly, renal insufficiency, which is common in liver transplant candidates, is closely associated with significant alterations in lipid metabolism. Therefore, it is necessary to determine the associations between both renal and hepatic function and PTHL.
The pathogenesis of PTHL remains to be elucidated. Possible factors which may increase the risk of PTHL include overweight or obesity, advanced age, and the use of immunosuppressive agents. Immunosuppressive drugs were considered to have a major effect on the development of metabolic syndromes in previous years. Today, immunosuppressive drugs are known to have a reduced impact on glucose or lipid metabolism. Low toxicity immunosuppressive protocols (e.g., early withdrawal of steroids and low-dose calcineurin inhibitors) have decreased the impact of immunosuppressive drugs on PTHL.
Over the past few years, most researchers have focused their attention on immunosuppressive protocols and obesity status. However, together with the application of lower toxicity immunosuppressive protocols, an increasing number of articles have shown that novel immunosuppressive therapy has less of an impact on glucose and lipid metabolism. Therefore, authors looked into the possibility that kidney and graft function may have a potential relationship with PTHL. From the results, renal and graft function were shown to correlate with lipid metabolism after living donor liver transplantation. Severe pre-transplant renal insufficiency may lead to long-term post-transplant renal dysfunction, and consequently result in PTHL, especially hypertriglyceridemia. Appropriate clinical treatment such as the prophylactic use of fibrates or statins may prevent PTHL in patients who develop early renal dysfunction or have longstanding pre-transplant renal dysfunction.
This study suggested that there was a correlation between renal function and PTHL, which may be of great help in better understanding the pathogenesis of PTHL, and as much as possible, preventing its development.
This is a good retrospective study. There were some limitations in this study, but were reasonably explained by the authors. In my opinion, this study is original and it is acceptable for publication.
Peer reviewers: Hatipoglu S, Department of Surgery, Inonu University Faculty of Medicine, 44000 Malatya, Turkey; Herrero JI, Liver Unit, University Clinic and CIBERehd, University of Navarra, Ave Pio XII 36, 31008 Pamplona, Spain; Mehmet Yilmaz, Department of General Surgery, Inonu University, School of Medicine, 44280 Malatya, Turkey
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Kobashigawa JA, Kasiske BL. Hyperlipidemia in solid organ transplantation. Transplantation. 1997;63:331-338. [PubMed] [Cited in This Article: ] |
| 2. | Dehghani SM, Taghavi SA, Eshraghian A, Gholami S, Imanieh MH, Bordbar MR, Malek-Hosseini SA. Hyperlipidemia in Iranian liver transplant recipients: prevalence and risk factors. J Gastroenterol. 2007;42:769-774. [PubMed] [Cited in This Article: ] |
| 3. | Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648-1654. [PubMed] [Cited in This Article: ] |
| 4. | Pfitzmann R, Nüssler NC, Hippler-Benscheidt M, Neuhaus R, Neuhaus P. Long-term results after liver transplantation. Transpl Int. 2008;21:234-246. [PubMed] [Cited in This Article: ] |
| 5. | Madhwal S, Atreja A, Albeldawdi M, Lopez R, Post A, Costa MA. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transpl. 2012;18:1140-1146. [PubMed] [Cited in This Article: ] |
| 6. | Faenza A, Fuga G, Nardo B, Donati G, Cianciolo G, Scolari MP, Stefoni S. Metabolic syndrome after kidney transplantation. Transplant Proc. 2007;39:1843-1846. [PubMed] [Cited in This Article: ] |
| 7. | Johnston SD, Morris JK, Cramb R, Gunson BK, Neuberger J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73:901-906. [PubMed] [Cited in This Article: ] |
| 8. | Toth PP. Drug treatment of hyperlipidaemia: a guide to the rational use of lipid-lowering drugs. Drugs. 2010;70:1363-1379. [PubMed] [Cited in This Article: ] |
| 9. | Pruthi J, Medkiff KA, Esrason KT, Donovan JA, Yoshida EM, Erb SR, Steinbrecher UP, Fong TL. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001;7:811-815. [PubMed] [Cited in This Article: ] |
| 10. | Anastácio LR, Ferreira LG, Ribeiro Hde S, Liboredo JC, Lima AS, Correia MI. Metabolic syndrome after liver transplantation: prevalence and predictive factors. Nutrition. 2011;27:931-937. [PubMed] [Cited in This Article: ] |
| 11. | Xu X, Ling Q, Wu J, Chen J, Gao F, Feng XN, Zheng SS. A novel prognostic model based on serum levels of total bilirubin and creatinine early after liver transplantation. Liver Int. 2007;27:816-824. [PubMed] [Cited in This Article: ] |
| 12. | Xu X, Ling Q, Zhang M, Gao F, He Z, You J, Zheng S. Outcome of patients with hepatorenal syndrome type 1 after liver transplantation: Hangzhou experience. Transplantation. 2009;87:1514-1519. [PubMed] [Cited in This Article: ] |
| 13. | Xu X, Ke QH, Shao ZX, Wu J, Chen J, Zhou L, Zheng SS. The value of serum alpha-fetoprotein in predicting tumor recurrence after liver transplantation for hepatocellular carcinoma. Dig Dis Sci. 2009;54:385-388. [PubMed] [Cited in This Article: ] |
| 14. | Xu X, Ling Q, Wei Q, Wu J, Gao F, He ZL, Zhou L, Zheng SS. An effective model for predicting acute kidney injury after liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:259-263. [PubMed] [Cited in This Article: ] |
| 15. | Xu X, Ling Q, He ZL, Gao F, Zheng SS. Post-transplant diabetes mellitus in liver transplantation: Hangzhou experience. Hepatobiliary Pancreat Dis Int. 2008;7:465-470. [PubMed] [Cited in This Article: ] |
| 16. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [PubMed] [Cited in This Article: ] |
| 17. | Deschênes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation. 1998;66:302-310. [PubMed] [Cited in This Article: ] |
| 18. | Gainza FJ, Valdivieso A, Quintanilla N, Errazti G, Gastaca M, Campo M, Lampreabe I, Ortiz-de-Urbina J. Evaluation of acute renal failure in the liver transplantation perioperative period: incidence and impact. Transplant Proc. 2002;34:250-251. [PubMed] [Cited in This Article: ] |
| 19. | Kaysen GA. Lipid and lipoprotein metabolism in chronic kidney disease. J Ren Nutr. 2009;19:73-77. [PubMed] [Cited in This Article: ] |
| 20. | Yamamoto S, Kon V. Mechanisms for increased cardiovascular disease in chronic kidney dysfunction. Curr Opin Nephrol Hypertens. 2009;18:181-188. [PubMed] [Cited in This Article: ] |
| 21. | Van Biesen W, De Bacquer D, Verbeke F, Delanghe J, Lameire N, Vanholder R. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J. 2007;28:478-483. [PubMed] [Cited in This Article: ] |
| 22. | Attman PO, Samuelsson O. Dyslipidemia of kidney disease. Curr Opin Lipidol. 2009;20:293-299. [PubMed] [Cited in This Article: ] |
| 23. | Prinsen BH, de Sain-van der Velden MG, de Koning EJ, Koomans HA, Berger R, Rabelink TJ. Hypertriglyceridemia in patients with chronic renal failure: possible mechanisms. Kidney Int Suppl. 2003;S121-S124. [PubMed] [Cited in This Article: ] |
| 24. | Delgado-Coello B, Briones-Orta MA, Macías-Silva M, Mas-Oliva J. Cholesterol: recapitulation of its active role during liver regeneration. Liver Int. 2011;31:1271-1284. [PubMed] [Cited in This Article: ] |
| 25. | Krycer JR, Brown AJ. Cross-talk between the androgen receptor and the liver X receptor: implications for cholesterol homeostasis. J Biol Chem. 2011;286:20637-20647. [PubMed] [Cited in This Article: ] |
| 26. | Ruiz-Rebollo ML, Sánchez-Antolín G, García-Pajares F, Fernández-Orcajo P, González-Sagrado M, Cítores-Pascual MA, Velicia-Llames R, Caro-Patón A. Risk of development of the metabolic syndrome after orthotopic liver transplantation. Transplant Proc. 2010;42:663-665. [PubMed] [Cited in This Article: ] |
| 27. | Li HY, Li B, Wei YG, Yan LN, Wen TF, Zhao JC, Xu MQ, Wang WT, Ma YK, Yang JY. Higher tacrolimus blood concentration is related to hyperlipidemia in living donor liver transplantation recipients. Dig Dis Sci. 2012;57:204-209. [PubMed] [Cited in This Article: ] |