Published online Feb 28, 2013. doi: 10.3748/wjg.v19.i8.1271
Revised: October 16, 2012
Accepted: November 11, 2012
Published online: February 28, 2013
AIM: To evaluate effective alternative antibiotics in treatment of cefotaxime-resistant spontaneous bacterial peritonitis.
METHODS: One hundred cirrhotic patients with spontaneous bacterial peritonitis [ascitic fluid polymorphonuclear cell count (PMNLs) ≥ 250 cells/mm3 at admission] were empirically treated with cefotaxime sodium 2 g/12 h and volume expansion by intravenous human albumin. All patients were subjected to history taking, complete examination, laboratory tests (including a complete blood cell count, prothrombin time, biochemical tests of liver and kidney function, and fresh urine sediment), chest X-ray, a diagnostic abdominal paracentesis, and the sample subjected to total and differential cell count, chemical examination, aerobic and anaerobic cultures. Patients were divided after 2 d by a second ascitic PMNL count into group I; patients sensitive to cefotaxime (n = 81), group II (n = 19); cases resistant to cefotaxime (less than 25% decrease in ascitic PMNL count). Patients of group II were randomly assigned into meropenem (n = 11) or levofloxacin (n = 8) subgroups. All patients performed an end of treatment ascitic PMNL count. Patients were considered improved when: PMNLs decreased to < 250 cells/mm3, no growth in previously positive culture cases, and improved clinical manifestations with at least 5 d of antibiotic therapy.
RESULTS: Age, sex, and Child classes showed no significant difference between group I and group II. Fever and abdominal pain were the most frequent manifestations and were reported in 82.7% and 80.2% of patients in group I and in 94.7% and 84.2% of patients in group II, respectively. Patients in group II had a more severe ascitic inflammatory response than group I and this was demonstrated by more ascitic lactate dehydrogenase (LDH) [median: 540 IU/L (range: 150-1200 IU/L) vs median: 240 IU/L (range: 180-500 IU/L), P = 0.000] and PMNL [median: 15 000 cell/mm3 (range: 957-23 822 cell/mm3) vs 3400 cell/mm3 (range: 695-26 400 cell/mm3), P = 0.000] counts. Ascitic fluid culture was positive in 32% of cases. Cefotaxime failed in 19% of patients; of these patients, 11 (100%) responded to meropenem and 6 (75%) responded to levofloxacin. Two patients with failed levofloxacin therapy were treated according to the in vitro culture and sensitivity (one case was treated with vancomycin and one case was treated with ampicillin/sulbactam). In group II the meropenem subgroup had higher LDH (range: 108-860 IU/L vs 120-491 IU/L, P = 0.042) and PMNL counts (range: 957-23 822 cell/mm3vs 957-15 222 cell/mm3, P = 0.000) at initiation of the alternative antibiotic therapy; there was no significant difference in the studied parameters between patients responsive to meropenem and patients responsive to levofloxacin at the end of therapy (mean ± SD: 316.01 ± 104.03 PMNLs/mm3vs 265.63 ± 69.61 PMNLs/mm3, P = 0.307). The isolated organisms found in group II were; enterococci, acinetobacter, expanded-spectrum β-lactamase producing Escherichia coli, β-lactamase producing Enterobacter and Staphylococcus aureus.
CONCLUSION: Empirical treatment with cefotaxime is effective in 81% of cases; meropenem is effective in cefotaxime-resistant cases.
- Citation: Badawy AA, Zaher TI, Sharaf SM, Emara MH, Shaheen NE, Aly TF. Effect of alternative antibiotics in treatment of cefotaxime resistant spontaneous bacterial peritonitis. World J Gastroenterol 2013; 19(8): 1271-1277
- URL: https://www.wjgnet.com/1007-9327/full/v19/i8/1271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i8.1271
Spontaneous bacterial peritonitis (SBP) is infection of ascitic fluid in cirrhotic patients. In 1985, results of a randomized trial demonstrated that cefotaxime, as a third-generation cephalosporin, achieved cure in SBP episodes for 85% of patients, compared to 56% of patients who received ampicillin plus tobramycin[1]. Since then, cefotaxime has become the empiric antibiotic of choice for the treatment of SBP. Mortality rates reduced from 90% when it was first described to around 20%-40%[2]. The widespread use of diagnostic paracentesis, as well as the prompt initiation of empiric antibiotic therapy, based on the results of ascitic polymorphonuclear (PMNL) cell count, has contributed in the improvement of survival in these patients[3]. Recent reports showed that resistance to third generation cephalosporins (including cefotaxime) is increasing[4,5] and this may increase the need to develop alternative antibiotics. The primary aim of this study was to evaluate effective alternative antibiotics in treatment of cefotaxime-resistant spontaneous bacterial peritonitis.
This prospective study was conducted at the Tropical Medicine Department, Zagazig University Hospitals, Zagazig, Sharkia Governorate, Egypt during the period from October 2010 to October 2011. This study was approved by the Ethical committee of our institution. Written informed consents were obtained from all patients. For patients with any grade of hepatic encephalopathy, the written informed consents were obtained from the first degree relatives.
All patients were inpatients and underwent diagnostic abdominal paracentesis with a differential leucocyte count, microbiological culture, and biochemical analysis at admission. Cefotaxime sodium 2 g/12 h (iv) was used as the initial empirical therapy because it is supported by the current guidelines as the first line of therapy for SBP[2]. It was subsequently maintained or replaced depending on the clinical course, a second abdominal paracentesis with PMNL count, and the in vitro susceptibility of the isolated microorganisms, up to a planned period of 5-7 d in a hospitalized basis.
After 48 h, a second abdominal paracentesis was done; cases with < 25% reduction in PMNL count were considered resistant to cefotaxime and were then randomly assigned to receive either meropenem 1 g/8 h (iv) or levofloxacin 1 g/12 h (iv drip). They were randomized by matching the sample (resistant group) and were classified into matched pairs; one from each pair was randomly enrolled into either the meropenem subgroup or the levofloxacin subgroup. One patient who was initially assigned to the levofloxacin subgroup was moved to the meropenem subgroup due to drug availability at the time of therapy. The final number was 11 patients in the meropenem subgroup and 8 in the levofloxacin subgroup.
The choice of these antibiotics was not only a matter of availability but also mainly due to previous reports of activity against bacterial species causing SBP[6,7] both in vitro[8] and in vivo[9] at national[10] and international[11] levels.
Five days after initiation of alternative antibiotic therapy an end of treatment diagnostic abdominal paracentesis was performed; cases with < 25% reduction in PMNL count from the second puncture counts were treated according to the results of in vitro culture and sensitivity that were available. All cases with initially positive ascitic fluid culture were re-cultured. Intravenous expansion was performed with human albumin 20 g iv infusion at the time of diagnosis and after 48 h.
All patients underwent a final diagnostic paracentesis with a differential leucocyte count at the end of therapy. The antibiotic dosage was adjusted to renal function throughout the treatment period. Diuretics were routinely discontinued at the time of diagnosis of SBP and therapeutic paracentesis was not allowed during the study.
All patients were subjected to history taking, complete clinical examination, laboratory tests (including a complete blood cell count, prothrombin time, biochemical tests of liver and kidney function, and fresh urine sediment), chest x-ray film, a diagnostic abdominal paracentesis, and the sample was subjected to total and differential cell count, chemical examination, aerobic and anaerobic culture. These were performed in all the cirrhotic patients with ascites on the day of admission and whenever they developed symptoms and signs suspicious for SBP (i.e., fever, change in mental status, abdominal pain, development of renal failure, hypotension, etc.) during the hospitalization period.
The ascitic fluid samples were collected under aseptic conditions in tubes containing ethylenediamine tetraacetic acid anticoagulant and then tested to determine white blood cell and PMNL counts by automated cell blood counter; chemical analysis for glucose, lactate dehydrogenase (LDH) and total protein were also done. All the specimens were analyzed within one hour. Moreover, 10 mL of ascitic fluid was inoculated directly at the patient’s bedside into aerobic and anaerobic blood culture bottles for bacteriological examination.
Ascitic fluid cultures were performed at the time of the initial paracentesis in the blood culture bottles (Remaux, France) at the bedside. All were incubated aerobically and anaerobically at 37 °C for 2 d before a subculture was done. Cases were considered negative after 7 d. All initially positive cases were re-cultured and cases were considered resolved from infection when PMNLs count had dropped to < 250/mm3 and all culture cases were negative.
Cirrhosis: Cirrhosis diagnosed based on clinical, biochemical, histological and/or radiological findings and Child class was assessed for each patient.
SBP: SBP was defined as the presence of ≥ 250 PMNLs/mm3 in the ascitic fluid[2].
Resolution of SBP: Resolution of SBP was defined as reduction of the elevated ascitic fluid PMNL count to < 250/mm3, negative culture in previously positive cases with resolution of the clinical manifestations and normalization of the impaired renal function.
Resistance: Resistance to empiric therapy was considered when < 25% reduction in PMNL count from the base line was achieved after 48 h[2].
Exclusion criteria: (1) Non-cirrhotic ascites (including malignant ascites); (2) Cases with secondary bacterial peritonitis; and (3) Bacterascites (i.e., positive ascitic fluid culture with < 250 neutrophils/mm3).
Data were checked, entered and analyzed using SPSS Version 15. Data were expressed as mean ± SD for quantitative variables, number and percentage for qualitative ones. χ2 or Fisher exact, t test and paired t test were used when appropriate. P < 0.05 was considered significant.
Baseline characteristics of patients are presented in Table 1. Age and gender were comparable in group I and group II. In group I, the numbers in Child class B and C were 21% and 79% while in group II they were 5.3% and 94.7%, respectively. This means that most cefotaxime resistance cases were associated with advanced liver disease.
| Variable | Group I (n = 81) | Group II(n = 19) | χ2 | P value |
| Gender | ||||
| Male | 48 (59.3) | 11 (57.9) | 0.01 | 0.91 |
| Female | 33 (40.7) | 8 (42.1) | ||
| Age (yr) mean ± SD | 49.4 ± 7.74 | 51.5 ± 8.08 | 1.04 | 0.29 |
| Abdominal pain | ||||
| Yes | 65 (80.2) | 16 (84.2) | 0.01 | 0.9 |
| No | 16 (19.8) | 3 (15.8) | ||
| Fever | ||||
| Yes | 67 (82.7) | 18 | 0.93 | 0.33 |
| No | 14 (17.3) | 1 (5.3) | ||
| Child Class | ||||
| B | 17 (21) | 1 (5.3) | 1.62 | 0.2 |
| C | 64 (79) | 18 (94.7) | ||
| Abdominal tenderness | ||||
| Yes | 43 (53.1) | 12 (63.2) | 0.11 | 0.427 |
| No | 38 (46.9) | 7 (36.8) | ||
| Upper GI bleeding | ||||
| Yes | 8 (9.9) | 4 (21.1) | 1.820 | 0.177 |
| No | 73 (90.1) | 15 (78.9) | ||
| Encephalopathy | ||||
| Yes | 25 (30.9) | 12 (63.2) | 6.885 | 0.009 |
| No | 56 (69.1) | 7 (36.8) | ||
| Asymptomatic | ||||
| Yes | 15 (18.5) | 0 (0.00) | 4.08 | 0.04 |
| No | 66 (81.5) | 19 (100) | ||
Fever and abdominal pain were the most frequent manifestations and were reported in 82.7% and 94.7% of patients in group I and in 80.2% and 84.2% of patients in group II, respectively. Asymptomatic patients were found in 18.5% of the cefotaxime sensitive group, but all patients of the resistant group presented with symptoms and signs. Regarding the laboratory parameters (Table 2), patients in group II had a greater peripheral blood leucocyte response than group I.
| Variable/mean ± SD | Group I (n = 81) | Group II (n = 19) | t | P value |
| Laboratory parameters | ||||
| INR | 1.87 ± 0.48 | 1.85 ± 0.57 | 0.091 | 0.927 |
| Albumin (g/dL) | 2.16 ± 0.32 | 2.23 ± 0.22 | 0.852 | 0.396 |
| Total bilirubin (mg/dL) | 5.45 ± 4.50 | 4.23 ± 2.60 | 1.133 | 0.260 |
| Direct bilirubin (mg/dL) | 3.44 ± 3.25 | 2.50 ± 1.95 | 1.202 | 0.232 |
| Creatinine (mg/dL) | 1.62 ± 0.85 | 1.80 ± 0.59 | 0.882 | 0.38 |
| Urea (mg/dL) | 83.87 ± 37.16 | 88.33 ± 42.99 | 0.457 | 0.649 |
| Potassium (mmol/L) | 3.76 ± 0.65 | 3.65 ± 0.79 | 0.586 | 0.559 |
| Sodium (mmol/L) | 130.36 ± 9.26 | 129.93 ± 10.53 | 0.178 | 0.859 |
| Platelets (× 10/mL) | 53.2 ± 20.1 | 54.6 ± 31.5 | 0.235 | 0.815 |
| Ascitic fluid parameters | ||||
| Glucose (mg/dL) | 121.05 ± 41.62 | 101.52 ± 53.032 | 1.744 | 0.084 |
| Protein (mg/dL) | 1389.95 ± 840.65 | 1553.63 ± 566.38 | 0.805 | 0.423 |
| LDH (IU/L) | 240 (180-500) | 540 (108-1200) | 4.406 | 0.000 |
| PMNLs (/mm3) | 3400 (695-264 00) | 15 000 (957-23 822) | 3.852 | 0.000 |
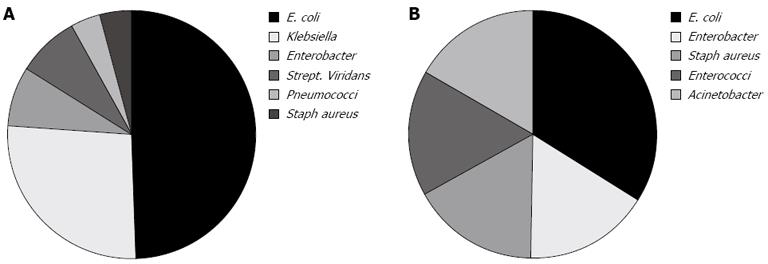
Ascitic fluid culture was positive in only 32% of cases. There were 26 ascitic fluid culture positive cases within group I (out of 81); the isolated organisms were Escherichia coli (E. coli) in 13 (50%), Klebsiella in 7 (27%), Enterobacter, and Strep. viridans each in 2 (8%), Pneumococci and S. aureus each in 1 (4%) (Figure 1A). There were 6 ascitic fluid culture positive cases within group II (out of 19); the isolated organisms were E. coli in 2 (33%), Enterobacter, S. aureus, Enterococci and Acinetobacter each in one (16%) (Figure 1B).
Data for all patients are presented in Tables 2 and 3. Patients in group II had a more severe ascitic inflammatory response than group I and this was demonstrated by the more ascitic LDH and PMNL counts. In group I, apart from the significant reduction in PMNL count, none of the biochemical parameters showed significant changes at the end of therapy. In group II the meropenem subgroup had higher LDH and PMNL count at initiation of the alternative antibiotic therapy; there was no significant difference in the studied parameters between patients responsive to meropenem and levofloxacin at the end of therapy.
| Variable | Meropenem subgroup | Levofloxacin subgroup | t | P value |
| (n = 11) | (n = 8) | |||
| At initiation of alternative therapy | ||||
| Glucose (mg/dL) | 100.45 ± 80.33 | 113.50 ± 9.58 | 0.390 | 0.702 |
| Protein (mg/dL) | 1850.00 ± 529.26 | 1360.83 ± 572.08 | 1.771 | 0.097 |
| LDH (IU/L) | 450 (108-1200) | 237 (120-831) | 2.223 | 0.042 |
| PMNLs/mm3 | 18 061 (957-23822) | 3540 (957-15222) | 3.622 | 0.000 |
| At end of therapy | ||||
| Glucose (mg/dL) | 102.81 ± 39.35 | 124.00 ± 29.17 | 1.150 | 0.268 |
| Protein (mg/dL) | 1660.00 ± 418.85 | 1163.33 ± 516.78 | 2.156 | 0.052 |
| PMNLs/mm3 | 316.01 ± 104.03 | 265.63 ± 69.61 | 1.056 | 0.307 |
Cases were treated for a median of 5-12 d. Cases in group I (n = 81) were treated by cefotaxime for 5-7 d, while patients in group II were treated as follows: the meropenem subgroup was treated for 5 d, the levofloxacin subgroup was treated for 5 d, 2 cases who were resistant to levofloxacin were treated with vancomycin in one case and ampicillin/sulbactam in one case, according to the in vitro culture and sensitivity results, for further 5 d and were recultured and examined by an end of treatment ascitic PMNL count before being considered cured.
The clinical and laboratory parameters of group II patients showed no major improvement with the initial empirical therapy; whereas noticeable improvements in many of the parameters were achieved when the alterative antibiotic therapy was given. This was obvious in hepatic encephalopathy (HE) which was encountered in 30.9% of patients in group I and 63.2% of patients in group II (grade II-III HE), where an improvement of consciousness level was achieved after antibiotic treatment of SBP. All patients of group II, in spite of starting treatment of HE, showed no major improvement with cefotaxime, but improvement of consciousness level occurred after the resolution of SBP by the alternative antibiotic therapy.
Cefotaxime as a standard treatment of SBP has been tried for many years with many reports of evolving resistance to cefotaxime and other third-generation cephalosporins in SBP[2,5]. Resistance to cefotaxime in this work is 19%, close to 21.5% recently reported by Ariza et al[5].
In this study cefotaxime resistance is not related to age or sex of patients, and the male predominance in our study is directly related to the higher rates of cirrhosis and risk of exposure and is in agreement with previous studies from other parts of the world[12,13].
Clinical manifestations of SBP are nonspecific; the most frequently encountered symptoms and signs are fever (69%) and abdominal pain (59%),[14]. This was the position in our study where fever and abdominal pain were the most frequent clinical manifestations.
However approximately 10% of patients with SBP are asymptomatic[14]; in our study asymptomatic patients made up 18.5% of the cefotaxime sensitive group, but all patients in the resistant group had clinical manifestations. This notion, together with the low yield of ascitic fluid culture, raised the value of PMNL count ≥ 250 cell/mm3 as the most important parameter for the diagnosis of SBP.
It is well known that infection in cirrhotic patients, especially SBP, precipitates HE and has been related to variceal bleeding both in terms of pathogenesis of portal pressure increase and severity of bleeding episodes[15] hence the related mortality was reduced by prompt antibiotic therapy[16] and this is confirmed by our study.
SBP occurs due to bacterial translocation, mainly in patients with advanced cirrhosis and severe liver functional damage[13,14,17]; this is confirmed in this study as SBP developed only in patients with Child classes B and C.
In this study, resistance to cefotaxime is associated with more advanced liver disease, because 94.7% and 5.3% of cases were Child C and B respectively. This notion needs further evaluation.
The low proportion of positive ascitic fluid cultures is probably due to the relatively low concentration of bacteria in the ascitic fluid as compared with the infections in other organic fluids (e.g., urine)[18]. For the same reason, a therapy based on the isolation of the responsible bacteria is seldom achievable and antibiotic treatment cannot be delayed to the moment when microbiological results are available[19]. That is why cefotaxime as an empirical therapy should be begun without delay.
In the cefotaxime sensitive group gram negative bacteria were the most frequent (22/26; 85%) and E. coli was the most predominant; however Gram positive bacteria were isolated in 4 patients. In the resistant group, bacteria were isolated in 6 patients; these results are closely similar to Taşkiran et al[20] study which revealed positive ascitic fluid culture in 29.4% of cases with predominantly Gram negative bacteria. Angeloni et al[12] revealed positive cultures in 30% of episodes with predominantly Gram negative bacteria; in these episodes the percentage of treatment failure of the initial therapy with cefotaxime was 32% and this result is similar to our findings.
In our patients, cefotaxime failed because the isolated organisms were intrinsically resistant to cefotaxime as Enterococci (one patient) and Acinetobacter spp (one patient) or were capable of degrading the expanded-spectrum cephalosporins as expanded-spectrum β-lactamase producing (ESBL) E. coli (two patients) or β-lactamase producing Enterobacter species (one patient) or bacteria with inherent insufficient susceptibility to cefotaxime, such as S. aureus (one patient). Our study supports previous reports[21] about the introduction of new organisms in the development of SBP.
The efficacy of empiric antibiotic treatment can rarely be based on the amelioration of symptoms or on microbiological results. Therefore, a reduction of PMNL count below 250 cell/mm3 or a 25% reduction of the initial value has been suggested as the main criterion for establishing the efficacy of antibiotics and the need for switching therapy. Our study confirms the validity of such an approach. Based on PMNL count, we identified the failure of the initial therapy on time.
Meropenem use was successful in 100% of resistant cases. Levofloxacin was successful in 75% of resistant cases, a result lower than reported for in vivo[9,22] and in vitro isolates where all aerobic isolates of SBP were sensitive to levofloxacin[23]. Other studies recommended meropenem use, not only in severe cases but also when ESBL producing E. coli is seen in the cultures and points to a relation between quinolone resistance and ESBL production[7,24]. Types of the isolated bacteria in group II of our study explain the efficacy of meropenem over levofloxacin.
Albumin use was suggested in SBP management[25], although it was not included in the treatment protocols. However the guidelines for the prevention and treatment of hepato-renal syndrome[26] suggested that albumin administration may reduce the incidence of renal failure and mortality in patients with SBP; consequently, all our patients received albumin. Some patients recorded a temporary increase in serum creatinine. All these patients showed improvement of kidney function with the use of proper antibiotics and resolution of SBP.
In conclusion, our study suggests that use of cefotaxime as the first line of treatment is considered valid, since a switch to another antibiotic was necessary only in 19% of our cases and we suggest meropenem as an effective alternative in resistant cases. This study also confirmed the value of a second abdominal paracentesis 48 h after initiation of empiric therapy to detect resistant cases early.
Spontaneous bacterial peritonitis is infection of ascitic fluid in cirrhotic patients with high mortality rates. Cefotaxime has become the empiric antibiotic of choice for the treatment since 1985. Mortality rates reduced from 90% when cefotaxime was first described to around 20%-40%. Recent reports showed that resistance to third generation cephalosporins (including cefotaxime) is increasing and this may increase the need to develop alternative antibiotics.
Eradication of ascitic fluid infection is the goal of antibiotic therapy. This study confirmed the validity of cefotaxime as an empiric first line therapy for spontaneous bacterial peritonitis and identified meropenem as an effective alternative in cases of cefotaxime resistance.
Cefotaxime as the empiric first line therapy for spontaneous bacterial peritonitis is effective in 81% of cases. Meropenem is effective in 100% of cefotaxime-resistant cases, while levofloxacin is effective in 75% of cefotaxime-resistant cases.
This article emphasises the value of meropenem and levofloxacin as effective alternatives to cefotaxime in resistant cases.
Spontaneous bacterial peritonitis is infection of ascitic fluid in patients with cirrhosis; Cefotaxime (a third generation cephalosporin) is utilized primarily for its activity against Gram-negative aerobic organisms. The drug exhibits moderate activity against Gram-positive bacteria. Meropenem is an ultra-broad spectrum injectable antibiotic used to treat a variety of infections. It is a beta lactam antibiotic and belongs to the subgroup of carbapenems; Levofloxacin is a synthetic chemotheraputic antibiotic which belongs to fluoroquinolone drug class and is used to treat severe or life-threatening bacterial infections or bacterial infections that have failed to respond to other antibiotic classes.
The clinical study focused on the effect of cefotaxime as the empiric first line therapy for spontaneous bacterial peritonitis and effectiveness of meropenem and levofloxacin as alternatives in case of cefotaxime resistance. This will be useful for future planning of therapy and clinical application.
P- Reviewer Butterworth J S- Editor Gou SX L- Editor O’Neill M E- Editor Zhang DN
| 1. | Felisart J, Rimola A, Arroyo V, Perez-Ayuso RM, Quintero E, Gines P, Rodes J. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457-462. [PubMed] [Cited in This Article: ] |
| 2. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [PubMed] [Cited in This Article: ] |
| 3. | Riggio O, Angeloni S. Ascitic fluid analysis for diagnosis and monitoring of spontaneous bacterial peritonitis. World J Gastroenterol. 2009;15:3845-3850. [PubMed] [Cited in This Article: ] |
| 4. | Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37:2-8. [PubMed] [Cited in This Article: ] |
| 5. | Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, Ariza J, Xiol X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56:825-832. [PubMed] [Cited in This Article: ] |
| 6. | Horinek E, Fish D. Spontaneous bacterial peritonitis. AACN Adv Crit Care. 2009;20:121-125; quiz 127. [PubMed] [Cited in This Article: ] |
| 7. | Heo J, Seo YS, Yim HJ, Hahn T, Park SH, Ahn SH, Park JY, Park JY, Kim MY, Park SK. Clinical features and prognosis of spontaneous bacterial peritonitis in korean patients with liver cirrhosis: a multicenter retrospective study. Gut Liver. 2009;3:197-204. [PubMed] [Cited in This Article: ] |
| 8. | Pfaller MA, Jones RN. A review of the in vitro activity of meropenem and comparative antimicrobial agents tested against 30,254 aerobic and anaerobic pathogens isolated world wide. Diagn Microbiol Infect Dis. 1997;28:157-163. [PubMed] [Cited in This Article: ] |
| 9. | Ahmad M, Ali AA, Mumtaz M, Iqbal J, Mughal AA. Spontaneous bacterial peritonitis; Microbiological analysis of ascitic fluid in patients with complicated liver cirrhosis. Professional Med J Jan. 2011;18:557-561. [Cited in This Article: ] |
| 10. | El-Bendary MM, Abdel-Aziz M, El-Sherbiny WA, Farag RE, El-Gilany A, Zaghloul MH. Spontaneous bacterial peritonitis: clinico-epidemiological and microbiological study. Benha Med J. 2009;26:287-306. [Cited in This Article: ] |
| 11. | Yakar T, Güçlü M, Serin E, Alişkan H, Husamettin E. A recent evaluation of empirical cephalosporin treatment and antibiotic resistance of changing bacterial profiles in spontaneous bacterial peritonitis. Dig Dis Sci. 2010;55:1149-1154. [PubMed] [Cited in This Article: ] |
| 12. | Angeloni S, Leboffe C, Parente A, Venditti M, Giordano A, Merli M, Riggio O. Efficacy of current guidelines for the treatment of spontaneous bacterial peritonitis in the clinical practice. World J Gastroenterol. 2008;14:2757-2762. [PubMed] [Cited in This Article: ] |
| 13. | Sun L, Zhang JV, Zhao J, Bai WT, Huang CX, Jia ZS, Lian JQ, Sun YT. Changes in the profiles of bacteria causing spontaneous bacterial peritonitis: A recent twelve-year study. Afr J Microbiol Res. 2010;4:527. [Cited in This Article: ] |
| 14. | Levison ME, Bush LM. Peritonitis and intraperitoneal abcesses. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier 2005; 927-951. [Cited in This Article: ] |
| 15. | Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet. 1999;353:139-142. [PubMed] [Cited in This Article: ] |
| 16. | Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655-1661. [PubMed] [Cited in This Article: ] |
| 17. | Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26-42. [PubMed] [Cited in This Article: ] |
| 18. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [PubMed] [Cited in This Article: ] |
| 19. | Hoefs JC. Spontaneous bacterial peritonitis: prevention and therapy. Hepatology. 1990;12:776-781. [PubMed] [Cited in This Article: ] |
| 20. | Taşkiran B, Colakoğlu O, Sözmen B, Unsal B, Aslan SL, Buyraç Z. Comparison of cefotaxime and ofloxacin in treatment of spontaneous bacterial peritonitis. Turk J Gastroenterol. 2004;15:34-38. [PubMed] [Cited in This Article: ] |
| 21. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [PubMed] [Cited in This Article: ] |
| 22. | Bert F, Noussair L, Lambert-Zechovsky N, Valla D. Viridans group streptococci: an underestimated cause of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Eur J Gastroenterol Hepatol. 2005;17:929-933. [PubMed] [Cited in This Article: ] |
| 23. | Cormican MG, Runyon BA, Jones RN. In vitro activity of levofloxacin and FK-037 against aerobic isolates from spontaneous bacterial peritonitis. J Chemother. 1995;7:197-200. [PubMed] [Cited in This Article: ] |
| 24. | Paterson DL. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs). Clin Microbiol Infect. 2000;6:460-463. [PubMed] [Cited in This Article: ] |
| 25. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [PubMed] [Cited in This Article: ] |
| 26. | Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |