Abstract
The study investigated the effects of adenovirus-mediated gene transfection of basic fibroblast growth factor (bFGF), bFGF combined with interleukin-1 receptor antagonist protein (IL-Ra) and/or insulin-like growth factor-1 (IGF-1) both in human osteoarthritis (OA) chondrocytes and rabbits OA model. Human OA chondrocytes were delivered by adenovirus-mediated bFGF, IL-Ra and IGF-1 vectors, respectively. Chondrocyte proliferation, glycosaminoglycan (GAG) content, expression of type II collagen, ADAMTS-5, MMP-13, MMP-3 and TIMP-1 were determined. Rabbit OA model was induced by anterior cruciate ligament transaction (ACLT) in knees. Adenoviral vectors encoding human bFGF, IL-Ra and IGF-1 were injected intraarticularly into the knee joints after ACLT. The effects of adenovirus- mediated gene transfection on rabbit OA were evaluated. In vitro, the transfected genes were expressed in cell supernatant of human OA chondrocytes. AdbFGF group significantly promoted chondrocyte proliferation, and increased GAG and type II collagen synthesis than in the OA group. As two or three genes were transfected in different combinations, there was significant enhancement on the GAG content, type II collagen synthesis, and TIMP-1 levels, while ADAMTS-5, MMP-13, and MMP-3 levels were reduced. In vivo, the transfected genes were expressed in synovial fluid of rabbits. Intraarticular delivery of bFGF enhanced the expression of type II collagen in cartilage and decreased cartilage Mankin score compared with the OA control group (P = 0.047; P < 0.01, respectively). Multiple-gene transfection in different combinations showed better results than bFGF transfection alone. This study suggests that bFGF gene transfection is effective in treating experimental OA. Multiple gene transfection has better biologic effects on OA.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a degenerative joint disease, which charactered by destruction of articular cartilage, remodeling of the subchondral bone, joint marginal osteophyte formation and synovitis. It is responsible for joint pain in middle-aged and older people and seriously influences their quality of life (De Ceuninck, 2007).
Among the current treatments for OA, orally administered analgesics and non-steroidal anti-inflammatory drugs can relieve pain, but their efficacy on OA progression is limited. Hyaluronic acid is easily applied by intraarticular injection. However, it has a short half-life and repeated intraarticular injections enhance the chances of joint infection (Lohmander et al., 1996). Advanced OA can only be managed by surgical replacement of joints, but there remain problems regarding the degree of invasion, cost and long-term prognosis (Berenbaum, 2008). For these reasons, an improved method that inhibits OA progression is needed. Currently, gene therapy has attracted much attention and has become a hot topic for investigation (Evans, 2005). It offers a radically new approach for treating OA (Evans et al., 2004).
Even though the etiology of OA is poorly understood, degeneration of articular cartilage occurs when catabolic activities exceed anabolic stimuli and the interplay between anabolic and catabolic activities is altered (Krasnokutsky et al., 2008). Thus, a selected gene of interest should inhibit extracellular matrix degradation and induce chondrocytes' biosynthesis of proteoglycan and collagen in vivo. Several cytokines play important roles in normal cartilage metabolism (Malemud, 2004; Blom et al., 2007).
Basic fibroblast growth factor (bFGF), also known as fibroblast growth factor 2 (FGF-2), belongs to the fibroblast growth factor (FGF) superfamily. It has been extensively studied in the literature, and found to be involved in numerous cellular functions by binding to and activation of cell-surface receptors in various cell types, including angiogenesis, cell proliferation, wound healing, limb formation, and tissue remodeling (Sorensen et al., 2006). Many studies have implied a potent anabolic effect of bFGF on cartilage homeostasis and suggested its use for cartilage injury and repair (Fujimoto et al., 1999; Yokoo et al., 2005; Nakajima et al., 2007). Moreover, Maniwa et al showed that bFGF could be the more potent chondrocyte mitogen among various growth factors (Kato et al., 1983, 1985; Maniwa et al., 2001), and could stimulate or stabilize the biosynthesis of cartilage matrix components (Yamamoto et al., 2004). However, there have been no reports regarding the effects of bFGF in gene therapy for OA. Theoretically, it is possible that bFGF inhibits OA progression due to its beneficial effects on cartilage, although the OA pathological process is different from that of cartilage injury.
The therapeutic effects of single gene delivery are limited (Zhang et al., 2004; Haupt et al., 2005). Interleukin-1 receptor antagonist protein (IL-1Ra) can inhibit cartilage matrix degeneration by blocking the adverse effects of interleukin-1 (IL-1) in OA (Frisbie et al., 2002). Insulin-like growth factor-1 (IGF-1) promotes the synthesis of cartilage matrix (Chubinskaya et al., 2007). These results show that bFGF, IL-1Ra and IGF-1 are potential candidate cytokines in gene therapy of OA. In the molecular network system to maintain cartilage homeostasis, a combination of these genes may have additive roles to achieve optimal therapeutic efficacy.
The aims of this study were to investigate the effects of adenovirus-mediated bFGF gene delivery on OA both in vitro in cultured human OA chondrocytes and in vivo in an OA animal model. The efficacies of the combination use of bFGF with IL-1Ra and/or IGF-1 gene delivery have also been explored.
Results
Viral particle titers and infectious titers
AdbFGF, AdIL-IRa and AdIGF-I particle titers were 2.8 × 1011 v.p./ml, 2.2 × 1011 v.p./ml and 3.9 × 1011 v.p./ml, respectively. AdbFGF, AdIL-IRa and AdIGF-I infectious titers were 1.1 × 1010 TCID50/ml, 4 × 109 TCID50/ml and 5.2 × 109 TCID50/ml, respectively.
In vitro results
Concentrations of bFGF, IL-1Ra and IGF-1 in cell supernatants
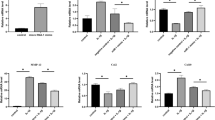
The expression levels of bFGF were higher in AdbFGF group (3137.33 ± 633.62 pg/ml), AdbFGF plus AdIL-1Ra group (3104.17 ± 1162.32 pg/ml), and AdbFGF plus AdIL-1Ra and AdIGF-1 group (2516.17 ± 404.66 pg/ml) than in OA group (359.83 ± 143.35 pg/ml) (Figure 1A). The expression levels of IL-1Ra were higher in AdbFGF plus AdIL-1Ra group (14159.50 ± 1638.29 pg/ml), AdbFG plus AdIL-1Ra and AdIGF-1 group (13074.50 ± 2989.23 pg/ml), and AdIL-1Ra plus AdIGF-1 group (14284.17 ± 2688.74 pg/ml) than in OA group (414.00 ± 126.28 pg/ml) (Figure 1B). The expression levels of IGF-1 were higher in AdbFGF plus AdIL-1Ra and AdIGF-1 group (21237.00 ± 3361.86 pg/ml), and AdIL-1Ra plus AdIGF-1 group (24064.50 ± 3777.33 pg/ml) than in OA group (474.67 ± 174.72 pg/ml) (Figure 1C).
Adenovirus-mediated human bFGF, IL-1Ra and IGF-1 gene expression levels in cell supernatant. Cultures of human OA chondrocytes were infected with AdbFGF (A), AdIL-1Ra (B) and AdIGF-1 (C) in different combinations at the indicated MOI. After 6 days, concentration of bFGF, IL-1Ra and IGF-1 was determined by ELISA. The bar represents the mean ± SD. (A): c vs. a, P = 0.001; c vs. b, P = 0.001; c vs. f, P = 0.001; d vs. a, P = 0.019; d vs. b, P = 0.023; d vs. f, P = 0.02; e vs. a, P < 0.01; e vs. b, P < 0.01; e vs. f, P < 0.01. (B): d vs. a, P < 0.01; d vs. b, P < 0.01; d vs. c, P < 0.01; e vs. a, P = 0.001; e vs. b, P = 0.001; e vs. c, P = 0.001; f vs. a, P = 0.001; f vs. b, P = 0.001; f vs. c, P = 0.001; (C): e vs. a, P < 0.01; e vs. b, P < 0.01; e vs. c, P < 0.01; e vs. d, P < 0.01; f vs. a, P < 0.01; f vs. b, P < 0.01; f vs. c, P < 0.01; f vs. d, P < 0.01.
Assay of cell proliferation
Chondrocyte proliferation was significantly increased in the AdbFGF group (0.67 ± 0.04), the AdbFGF plus AdIL-1Ra group (0.61 ± 0.03), the AdbFGF plus AdIL-1Ra and AdIGF-1 group (0.59 ± 0.03) and the AdIL-1Ra plus AdIGF-1 group (0.55 ± 0.04) compared with the OA group (0.40 ± 0.03) (Figure 2). Morerove, chondrocyte proliferation was significantly higher in the AdbFGF group than in the AdbFGF plus AdIL-1Ra group, the AdbFGF plus AdIL-1Ra and AdIGF-1 group, and the AdIL-1Ra plus AdIGF-1 group (Figure 2). There was no significant difference of cell proliferation between the AdEGFP group and OA group.
Glycosaminoglycan (GAG) contents in cell supernatants
The expression levels of GAG were higher in AdbFGF group (6.63 ± 0.93 µg/ml), the AdbFGF plus AdIL-1Ra group (9.05 ± 1.15 µg/ml), the AdbFGF plus AdIL-1Ra and AdIGF-1 group (11.93 ± 1.98 µg/ml), and the AdIL-1Ra plus AdIGF-1 group (8.98 ± 0.83 µg/ml) compared with the OA group (3.45 ± 1.20 µg/ml) (Figure 3). The increase in the AdbFGF plus AdIL-1Ra group, the AdbFGF plus AdIL-1Ra and AdIGF-1 group, and the AdIL-1Ra plus AdIGF-1 group was more significant compared with the AdbFGF group. Meanwhile, the expression levels of GAG were higher in the AdbFGF plus AdIL-1Ra and AdIGF-1 group than in the AdbFGF plus AdIL-1Ra group, and the AdIL-1Ra plus AdIGF-1 group, respectively (Figure 3).
Effects of AdbFGF, AdIL-1Ra and AdIGF-1 in different combinations on GAG synthesis in human OA chondrocytes. GAG content in cell supernatants was measured using DMMB dye binding assay. Each value represents the mean ± SD. c vs. a, P < 0.01; d vs. a, P < 0.01; e vs. a, P < 0.01; f vs. a; P < 0.01; d vs. c, P = 0.002; e vs. c, P < 0.01; f vs. c, P = 0.002; e vs. d, P < 0.01; e vs. f, P < 0.01.
Western blot for ADAMTS-5, MMP-13, MMP-3, TIMP-1 and collagen II
Compared with the OA group, Type II collagen and TIMP-1 expressions were increased and ADAMTS-5 expression was decreased statistically in the AdbFGF group, the AdbFGF plus AdIL-1Ra group, the AdbFGF plus AdIL-1Ra and AdIGF-1 group and the AdIL-1Ra plus AdIGF-1 group (Figure 4). Compared with the AdbFGF group, type II collagen and TIMP-1 expressions in the combined gene transfection groups were significantly increased while ADAMTS-5, MMP-13 and MMP-3 expressions were significantly decreased. Moreover, in the AdbFGF plus AdIL-1Ra and AdIGF-1 group, the expressions of type II collagen and TIMP-1 were statistically higher and ADAMTS-5, MMP-13, MMP-3 expression were significantly lower than in the AdbFGF plus AdIL-1Ra group. There was no significant difference as for ADAMTS-5, MMP-13, MMP-3, TIMP-1 and type II collagen expressions between the AdEGFP group and OA group (Table 1).
In vivo results
Levels of human bFGF, IL-1Ra and IGF-1 in synovial fluids
The expressions of human bFGF, IL-1Ra and IGF-1 were monitored in synovial fluid from knee joints of rabbit OA model by ELISA assays (the ELISA kits did not recognize rabbit endogenous bFGF, IL-1Ra and IGF-1 proteins). In the third, fourth and fifth animal groups that received AdbFGF, the respective average values of exogenous bFGF were (248.08 ± 57.07) pg/ml, (207.36 ± 32.87) pg/ml and (181.36 ± 42.08) pg/ml (Figure 5A). In the fourth, fifth and sixth groups that received AdIL-1Ra, the respective average values of exogenous IL-1Ra were (821.40 ± 126.06) pg/ml, (1390.82 ± 227.38) pg/ml and (1476.70 ± 165.39) pg/ml (Figure 5B). In the fifth and sixth groups that received AdIGF-1, the respective average values of exogenous IGF-1 were (2257.98 ± 383.30) pg/ml and (2658.26 ± 430.94) pg/ml (Figure 5C).
Adenovirus-mediated human bFGF, IL-1Ra and IGF-1 gene expression in synovial fluids from knees of rabbit OA. Concentration of exogenous bFGF (A), IL-1Ra (B) and IGF-1 (C) was determined by ELISA. The ELISA kits used in this study did not recognize the rabbit endogenous bFGF, IL-1Ra and IGF-1 proteins. Each value represents the mean ± SD. The expression of all transgenes was high following adenoviral transfection in synovial fluids.
GAG levels in synovial fluids
The level of GAG was markedly higher in the OA group (69.96 ± 8.32 µg/ml) than in the sham operated group (32.23 ± 3.76 µg/ml) (Figure 6). Compared with the OA group, the GAG contents were significantly decreased in the AdbFGF plus AdIL-1Ra group (54.95 ± 6.98 µg/ml), the AdbFGF plus AdIL-1Ra and AdIGF-1 group (50.28 ± 6.12 µg/ml) and the AdIL-1Ra plus AdIGF-1 group (48.33 ± 8.52 µg/ml) (Figure 6). The decrease of GAG in the AdbFGF plus AdIL-1Ra group, the AdbFGF plus AdIL-1Ra and AdIGF-1 group, and the AdIL-1Ra plus AdIGF-1 group was more significant than in the AdbFGF group. (Figure 6).
Concentration of GAG in synovial fluids from knees of rabbit OA. GAG was released into joint cavity from degraded cartilage matrix. GAG content in synovial fluids was measured using DMMB dye binding assay. Each value represents the mean ± SD. b vs. a, P < 0.01; c vs. b, P = ns; d vs. b, P = 0.004; e vs. b, P < 0.01; f vs. b, P < 0.01; d vs. c, P = 0.019; e vs. c, P = 0.002; f vs. c, P = 0.001. e vs. d, P = ns; e vs. f, P = ns. ns: not significant.
mRNA expressions of type II collagen in cartilage
As for expression of type II collagen mRNA, all the groups showed significant increases compared with the OA group. All of the combined gene transfection groups had significant increases compared with AdbFGF group. Moreover, the expression levels of type II collagen mRNA were higher in the AdbFGF plus AdIL-1Ra and AdIGF-1 group than in the AdbFGF plus AdIL-1Ra group (Figure 7).
The expression levels of type II collagen mRNA were determined by quantitative PCR. Results represent the mean ± S.D. Type II collagen mRNA expression levels were normalized to the expression in OA group in which type II collagen mRNA expression levels were arbitrarily set to 1.0. a vs. b, P < 0.01; c vs. b, P = 0.047; d vs. b, P = 0.001; e vs. b, P < 0.01; f vs. b, P < 0.01; d vs. c, P = 0.043; e vs. c, P = 0.001; f vs. c, P = 0.014. e vs. d, P = 0.046; e vs. f, P = ns. ns: not significant.
Histological examination of cartilage
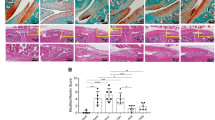
In the sham operated group, the articular cartilage surface was generally smooth, and the laminar structure was clear. However, the articular cartilage surface in the OA group was rough and had some superficial leakage and ulcers (Figure 8A). Safranin O staining for proteoglycans showed a distinct reduction of these compounds in the cartilage matrix (Figure 8B). After exogenous genes were transfected, the cartilage lesions and matrix degradation were less evident. Based on the Mankin scale, the severity of OA in AdbFGF group (6.00 ± 0.71), the AdbFGF plus AdIL-1Ra group (4.60 ± 1.14), the AdbFGF plus AdIL-1Ra and AdIGF-1 group (3.80 ± 0.84), and the AdIL-1Ra plus AdIGF-1 group (4.20 ± 1.10) was decreased than in OA group (8.60 ± 1.14) (Figure 8C). Compared with AdbFGF group, the combined gene transfection groups (the AdbFGF plus AdIL-1Ra group, the AdbFGF plus AdIL-1Ra and AdIGF-1 group, and the AdIL-1Ra plus AdIGF-1 group) markedly decreased Mankin scores (Figure 8C).
Histologic analysis and quantitative assessment of cartilage change in rabbits. Sagittal medial femoral condyles of the sections were stained with HE (A) and Safranin O (B). Data shown are representative microscopic images of knees from rabbits in the groups (original magnification × 100). Histologic quantitative assessment was performed by Mankin's score (C). Each value represents the mean ± SD. b vs. a, P < 0.01; c vs. b, P < 0.01; d vs. b, P < 0.01; e vs. b, P < 0.01; f vs. b, P < 0.01; d vs. c, P = 0.027; e vs. c, P = 0.001; f vs. c, P = 0.006. e vs. d, P = ns; e vs. f, P = ns. ns: not significant.
Discussion
This study shows that introduction of bFGF by transfection has a protective effect both on cultured human OA chondrocytes and on cartilage degradation in rabbit OA model. The in vitro results revealed that bFGF not only promoted chondrocyte proliferation in OA, but also enhanced GAG and type II collagen synthesis. Histological analysis showed that bFGF lowered the Mankin score of OA cartilage, promoted type II collagen synthesis in the matrix. The result of safranin O staining indicated that bFGF increased proteoglycan synthesis based on the in vitro finding for GAG.
Our findings corroborated the findings of Inoue et al. (2006) in a rabbit OA model. Sustained release of bFGF into the joint had therapeutic effects on OA development by the method of controlled-release gelatin hydrogel microspheres (Inoue et al., 2006). Meanwhile, some studies indicated that bFGF could be applied in the treatments for injured cartilage (Cuevas et al., 1988; Fujimoto et al., 1999; Weisser et al., 2001; Yamamoto et al., 2004) and suggested bFGF was potential for repair and regeneration of cartilage defects. Miyakosi et al. also reported that bFGF with hyaluronic acid had positive biological effect on osteochondral repair (Miyakosi et al., 2005). Hiraide et al. (2005) documented cartilage repair in an in vivo model of rabbit knee cartilage defects using an adeno-associated virus (AAV) to transport the bFGF gene into knee synovial tissue. Nevertheless, Sah et al. (1994) declared that low dose of bFGF could stimulate cell mitotic activity, synthesis of proteoglycan and collagen, while high dose of bFGF could inhibit mitotic activity. Hence, bFGF probably elicited dose-dependent and bi-directional effects on mitotic activity and anabolic processes. In current study, the biological effect of bFGF on OA was demonstrated by the method of adenovirus mediated gene delivery. Adenovirus mediated gene delivery has several advantages, including good biological safety, high transduction efficiency towards a wide variety of dividing and non-dividing cells, comparative ease with which high-titer preparations can be obtained (Kawabata et al., 2006; Shirakawa, 2009).
However, in this study, the level of exogenous bFGF in cell supernatants and synovial fluids were lower than that of IL-1Ra and IGF-1. A possible consequence of this is that the extent of the biologic effect of bFGF has been partly diminished. The low bFGF expression level is related to a lack of a secretion signal sequence and its insufficient extracellular secretion (Backhaus et al., 2004). Sorensen et al. (2006) found that bFGF could be produced as distinct isoforms by alternative initiation of translation on a single mRNA (i.e., high molecular weight and low molecular weight bFGF). The high molecular weight bFGF isoform was predominantly transported to the nucleus where it regulated cell growth or behavior in an intracrine fashion. Low molecular weight (18 kDa) bFGF could be secreted into the extracellular medium where it acted as a conventional growth factor by binding to and activating cell-surface receptors. Then, following receptor-mediated endocytosis, the exogenous bFGF could be transported to the nuclei of target cells and exerted its biologic effects (Taverna et al., 2003; Zehe et al., 2006). In our current study, exogenous bFGF expression was detected in cell supernatant and synovial fluid, although the amount was only about 1/10 to 1/5 of the other factors. It should be synthesized and secreted by transfected chondrocytes in vitro and synovial cells and exposed chondrocytes in cartilage in vivo. All these results show that bFGF could translocate across the cell membrane and exert its biologic effect. Thus, we believe that our findings are of interest since adenovirus-mediated bFGF gene delivery could provide a potential application for treating OA. However, how to increase the expression level and determine appropriate concentration of bFGF remains a problem that needs to be resolved.
When OA occurs, cartilage matrix degeneration is the primary process initiated by aggrecanase, metalloproteases and several cytokines, such as IL-1 and tumor necrosis factor-α (TNF-α) (Ge et al., 2006; Martel-Pelletier et al., 2008; Fosang et al., 2010; Piecha et al., 2010). Cartilage matrix degeneration causes the release of GAG into synovial fluids. Thus, changes of GAG levels in cartilage or synovial fluids reflect the extent of catabolic activity in the cartilage matrix (Alwan et al., 1991; Poole et al., 1994). In this study, bFGF alone did not reduce GAG content in synovial fluid as expected while the other factors took effects. And also, bFGF did not show any beneficial effect on MMP-13 and MMP-3 which are related to cartilage matrix degradation. Thus, we hypothesize that if bFGF alone is applied for gene therapy of OA, its effectiveness would be limited to some extent though it has strong effect on promoting chondrocyte proliferation and also it is able to increase GAG and type II collagen synthesis and the TIMP-1 expression, decrease ADAMTS-5 expression in the matrix (Sawaji et al., 2008; Chia et al., 2009).
Researches have shown that single cytokine or growth factor alone has a limited effect on OA (Zhang et al., 2004; Haupt et al., 2005). This is why combination of gene transfection for OA treatment has attracted extensive attention (Zhang et al., 2004; Haupt et al., 2005). Haupt et al. (2005) found adenovirus-mediated gene therapy combining IGF-1 and IL-1Ra could promote the healing of cartilage injury in degenerative joint diseases by the method of co-culture in cartilage explants and synovial monolayer medium, and suggested the benefit of combination therapy for cartilage healing in degenerative joint diseases. In vitro, our data showed that the three-gene transfection (bFGF combined with IL-1Ra and IGF-1) significantly reduced expression levels of ADAMTS-5, MMP-13 and MMP-3, and increased TIMP-1 level compared with bFGF alone or the two-gene transfections. Additionally, the three-gene combination also promoted type II collagen and GAG synthesis in matrix to greater degrees compared with bFGF alone or the two-gene transfections. And the findings in vivo corroborated those of in vitro. One possible mechanism is that IL-1Ra competitively binds to the interleukin-1 receptor and, consequently, blocks the production of aggrecanase and MMPs, thus avoiding the degeneration of proteoglycans and collagen of matrix (Pelletier et al., 1997; Zhang et al., 2004; Fosang et al., 2010). On the other hand, IGF-1 not only inhibits the expressions of MMPs production, thus preventing matrix degradation, but also promotes the synthesis of GAG and type II collagen, which is beneficial for cartilage repair (Zhang et al., 2009). Hence, gene transfection of bFGF combined with IL-1Ra and IGF-1 could have a synergistic effect to achieve optimal therapeutic efficacy. They resemble and are close to the cellular environment involving the interactions of multiple biologic factors in the body. Among the different combinations of multiple exogenous gene transfection, it seems that the three-gene combination of AdbFGF plus AdIL-1Ra and AdIGF-1 has the best biologic effects on OA, since it is the strongest one in promoting GAG synthesis of chondrocyte, increasing TIMP-1 expression and reducing ADAMTS-5 and MMP-13 expression and it has the similar effects to those of the other combinations in the aspects of promoting cell proliferation and type II collagen synthesis, reducing MMP-3 level and lowering Mankin score. Whether this finding indicates the more genes are transfected, the better result could be gained still needs to be investigated. For this, there may be a long way to go to reach the final conclusion.
Regarding the manner of in vivo gene delivery, we adopted local intra-articular injection of adenoviral vectors in vivo (Ghivizzani et al., 2001; Hsieh et al., 2010). In previous studies, some researchers have chosen ex vivo techniques, as the compact cartilage matrix might prevent the vectors from reaching chondrocytes (Evans et al., 2006). However, our data showed that extensive destruction of cartilage matrix, chondrocyte exposure to the joint cavity and osteophyte formation were found in the OA knee. These observations were similar to those in the literature (Punzi et al., 2005; van der Kraan et al., 2007). Therefore, it was possible for the adenoviral vectors to directly transfect the exposed chondrocytes. The synovial tissue is loose and synoviocytes on the surface of the synovium are easier to be transfected than chondrocytes embedded within the cartilage matrix. Synoviocytes are the predominant targets for adenoviral vector transfection. Compared with ex vivo techniques, intraarticular adenoviral vector injection exerts its effects both directly and indirectly. On one hand, after transfection, the expression product could be secreted by the synovium into synovial fluid in a joint cavity. As material exchange occurs between synovial fluid and cartilage, the expressed protein enters articular cartilage and subsequently influences cartilage metabolism and decreases OA progression. On the other hand, adenoviral vectors could directly target exposed chondrocytes and the expression product manufactured by the chondrocytes would exert its effect in situ on the chondrocyte itself and the matrix in a more direct manner than that produced in the synovium. The gene expressions in both synoviocytes and chondrocytes would have an additive, protective effect on the progression of OA.
In conclusion, our findings show that bFGF may be a suitable factor for the treatment of experimental OA by enhancing chondrocyte proliferation, proteoglycan synthesis and type II collagen expression. Meanwhile, the different combinations of multiple exogenous gene transfection has better biologic effects than bFGF alone. Further studies are necessary to explore the synergistic beneficial effects of selected genes in OA.
Methods
Construction of adenoviral vectors
The vectors used to produce adenovirus were replication-defective human adenovirus type 5 with complete deletion of the E1a and E1b regions and partial deletion of the E3 region of the viral genome. To generate Adenoviral bFGF, IL-1Ra and IGF-1, the human entire coding sequence of bFGF (468 bp), IL-1Ra (534 bp) and IGF-1 (462 bp) under the control of the cytomegalovirus (CMV) promoter was inserted into an adenoviral plasmid containing an enhanced green fluorescent protein (EGFP), respectively. The resulting vectors were designated AdbFGF, AdIL-1Ra and AdIGF-1, respectively. Recombinant viruses were propagated in human embryonic kidney 293 cells purchased from the American Type Culture Collection (ATCC, Manassas, VA) (Hardy et al., 1997; Hillgenberg et al., 2006). Viral particles were lysed from the packaging cell line and purified using 3 sequential cesium chloride gradients. Viral bands were then dialyzed in a sucrose buffer to remove remaining salt and stored at -80℃ until use. Viral particle titers were determined by fluorescence spectrophotometer F-4500 (Hitachi, Tokyo, Japan) (Mi et al., 2000). The values of virus infectious titers were measured by the method of 50% tissue culture infectious doses (TCID50/ml) (Lin et al., 2010).
In vitro experiment
Tissue collection
Cartilage tissues were obtained from 6 OA patients (aged 49-76 yr, 2 males and 4 females) who underwent total knee replacement at Zhongnan Hospital, Wuhan University. The patients met the American College of Rheumatology classification criteria for the diagnosis of osteoarthritis (Wu et al., 2005). Full ethical consent was obtained from all patients and the study was granted ethical approval by the Medical Ethical Committee of Zhongnan Hospital, Wuhan University.
Cell isolation and transfection
Cartilage was separated from underlying bone and connective tissue and digested for 6-8 h in 0.2% collagenase type II (Worthington, Lakewood, NJ) in Dulbecco's modified eagle's medium (DMEM) medium. Chondrocytes were cultured in DMEM medium with 10% fetal bovine serum at 37℃ with 5% CO2. The second passage of chondrocytes was used for the following study (Hendriks et al., 2006).
Adenoviral vectors containing bFGF, IL-IRa and IGF-I were transfected into human OA chondrocytes at multiplicity of infection (MOI) of 0, 50, 100, 150, 200, 250, 300, respectively. Cells were cultured at 37℃ with 5% CO2. After 48 h, cells were harvested and used to detected EGFP expression by flow cytometry, and cells were also visible by immunofluorescence microscopy (Supplemental Data Figure S1). The optimal MOIs value (AdbFGF: 150, AdIL-1Ra: 100 and AdIGF-1: 100) was determined by the percentage of live infected cells expressing GFP. Each experiment was repeated a minimum of three times.
Chondrocytes were divided into six groups: (1) OA chondrocytes without transfection; (2) OA chondrocytes transfected with AdEGFP at 100 MOI; (3) OA chondrocytes transfected with AdbFGF at 150 MOI; (4) OA chondrocytes transfected with AdbFGF (150 MOI) plus AdIL-1Ra (100 MOI); (5) OA chondrocytes transfected with AdbFGF (150 MOI) plus AdIL-1Ra (100 MOI) and AdIGF-1 (100 MOI); (6) OA chondrocytes transfected with AdIL-1Ra (100 MOI) plus AdIGF-1 (100 MOI). Transfection was performed in 600 µl of DMEM at 37℃ with 5% CO2 for 4 h. Following transfection, chondrocytes were incubated with serum-free medium containing 5 ng/ml recombinant human interleukin-lβ (R&D Systems, inneapolis, MN, USA) at 37℃ with 5% CO2 for a further 6 days (Haupt et al., 2005).
Concentrations of bFGF, IL-1Ra and IGF-1 in cell supernatants
The concentrations of bFGF, IL-1Ra and IGF-1 in cell supernatants at the 6th day after transfection were determined using enzyme linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocols.
Assay of cell proliferation
Seventy two hours after transfection, the culture medium was replaced with DMEM containing 0.5 mg/ml 3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and the chondrocytes were incubated at 37℃ with 5% CO2 for 4 h. The resulting formazan were dissolved in dimethylsulphoxide and absorbance was measured at 570 nm with a microplate reader (Shimadzu, Kyoto, Japan).
GAG contents in cell supernatants
The dimethylmethylene blue (DMMB) spectrophotometric assay was used to determine the GAG contents in cell supernatants (Barbosa et al., 2003). Cell supernatants were digested in 0.5 mg/ml papain for 2 h at 65℃. Following digestion, they were centrifuged at 1,500 g for 8 min. Then, DMMB solution (Sigma, St. Louis, MO) was added to the digested cell supernatants. The absorbance was measured at 525 nm using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan). A standard curve was derived from mixed-isomer shark chondroitin sulfate, and the GAG content was calculated.
Western blot for ADAMTS-5, MMP-13, MMP-3, TIMP-1 and collagen II
Proteins were extracted from harvested chondrocytes. Protein concentrations were determined using a BCA assay (Sigma, St. Louis, MO). Samples of 50 µl protein were separated on 12% sodium dodecyl sulphate-polyacrylamide gels before transferring to polyvinylidene difluoride membranes. Membranes were blocked with 5% non-fat milk powder and incubated with rabbit anti-ADAMTS-5 (1:250), MMP-13 (1:200), MMP-3 (1:200), TIMP-1 (1:100) and type II collagen (1:150) respectively (Shanghai Bluegene Biotech CO, LTD, China), and then incubated in a 1/4000 dilution with horseradish peroxidase-conjugated secondary antibodies (mouse anti-rabbit IgG, Shanghai Bluegene Biotech CO, LTD, China). The proteins were visualized by ECL detection (Amersham Pharmacia Biotech) following the manufacturer's instructions. β-actin was used as an internal control. Immunoblots were further semi-quantitatively analysed using the computer program 'Quantity one' (Bio-Rad, Munich, Germany). The mean optical density represented the relative expression levels of protein. Experiments were performed in triplicate.
In vivo experiment
Animal and experimental arthritis
New Zealand white rabbits (2.0-2.5 kg) were obtained from the Laboratory Animal Center of Wuhan University (Wuhan, Hubei, China). Animal care and treatment were in accordance with the guidelines of the Laboratory Animal Management and Review Committee of Wuhan University. The study was approved by the Medical Ethical Committee of Zhongnan Hospital, Wuhan University. Thirty rabbits were randomly divided into 6 groups of 5 rabbits each. The first group only had the right joint cavity opened and then the incision was sutured (sham operated group). The second was the OA model control group. The third, fourth, fifth and sixth groups underwent gene delivery. OA was induced by unilateral transections of the anterior cruciate ligaments (ACLT) and excisions of medial menisci in the right knees (Yoshioka et al., 1996).
Animals were anesthetized with pentobarbital (30 mg/kg, i.p.). Following surgery, the rabbits were housed in regular individual cages and closely monitored for infections and other complications. All rabbits were provoked to exercise for 4 h each day. Six weeks later, the sham and OA model groups received 1 ml of 0.9% (w/v) physiological saline by intraarticular injection. The third group received 1 × 108 plaque-forming units (pfu) of AdbFGF in the right knees. The fourth group received 1 × 108 pfu each of AdbFGF plus AdIL-1Ra. The fifth group received 1 × 108 pfu each of AdbFGF plus AdIL-1Ra and AdIGF-1. The sixth group received 1 × 108 pfu each of AdIL-1Ra plus AdIGF-1. Three weeks after adenovirus injections, the rabbits were sacrificed. Knees were washed with 1 ml of 0.9% (w/v) physiological saline solution through the patellar tendon. After centrifugation, synovial fluid washes were stored at -80℃ until analysis.
Concentrations of bFGF, IL-1Ra and IGF-1 in synovial fluids
The concentrations of exogenous bFGF, IL-1Ra and IGF-1 in lavage fluids were determined using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocols.
GAG contents in synovial fluids
The DMMB spectrophotometric assay was used to determine the GAG contents in synovial fluids. Protocol was as that for in vitro study.
Quantitative PCR of type II collagen mRNA
Total RNA was extracted from fresh cartilage tissue of the medial tibial plateau from right knees of rabbits with Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction. A total of 1 µg purified RNA was reversely transcribed into cDNA using a First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON, Canada).
The expression levels of type II collagen mRNA were detected by quantitative PCR analysator with a Rotor-Gene 3000 system (Corbett research, Concorde, NSW, Australia) using the Quantitect SYBR Green PCR Kit (Takara, Shiga, Japan) following the manufacturer's guidelines, employing the β-actin gene as a reference gene. The following primer pairs were used: Collagen II forward 5'-GCTCAAGTCCCTCAACAACCAG-3' and reverse 5'-GGTCTATCCAGTAGTCACCGCTCT-3', β-actin forward 5'-CGAGATCGTGCGGGACATs-3' and reverse 5'-CAGGAAGGAGGGCTGGAAC-3'. The quantitative PCR reaction was carried out in a 25 µl volume containing 2 × SYBR® Premix Ex Taq™ (Takara, Shiga, Japan) 12.5 µl, 0.2 µM of each primer, and 1 µl cDNA. PCR condition was as follows: an initial denaturation at 95℃ for 2 min, followed by 40 cycles of 95℃ for 15 s, 61℃ for 15 s, and 72℃ for 45 s, and final extension at 72℃ for 10 min. Detection of the Ct value of fluorescent product was done at the extension period using the Rotor-Gene 3000 accompanying quantification analysis software. Gene expressions of type II collagen in all samples were analyzed by applying the 2-ΔΔCt relative quantification method (Livak et al., 2001). To compare type II collagen mRNA expression levels in cartilage tissue among OA group, AdbFGF group, AdbFGF and AdIL-1Ra group, AdbFGF plus AdIL-1Ra and AdIGF-1 group, and AdIL-1Ra and AdIGF-1 group, type II collagen mRNA expression levels in OA group was arbitrarily set to 1.0.
Histological examination of cartilage
Following dissection, the samples of medial femoral condyles were fixed in 4% (v/v) formaldehyde and embedded into paraffin. Histological evaluation was performed on sagittal sections of cartilage from the lesion areas of medial femoral condyles. Serial sections (5 µm thick) were cut and stained with hematoxylin and eosin (HE) and Safranin O. The severity of OA lesions was scored from 0 to 14 according to the Mankin scale (Mankin et al., 1971).
Statistical analysis
The statistical analysis was done by SPSS 13.0 software (SPSS Inc., Chicago). The normal distribution of each data group was assessed with the Shapiro-Wilk test. For variables showing a normal distribution, comparisons between groups were performed with one-way ANOVA analysis, the data were reported as means ± standard deviation (SD). Statistical significance was defined by the value of P < 0.05.
Abbreviations
- ACLT:
-
anterior cruciate ligament transaction
- ADAMTS:
-
a disintegrin and metalloproteinase with thrombospondin motifs
- bFGF:
-
basic fibroblast growth factor
- Ct:
-
threshold cycle
- DMMB:
-
dimethylmethylene blue
- ECM:
-
extracellular matrix
- GAG:
-
glycosaminoglycan
- IGF-1:
-
insulin-like growth factor-1
- IL-1Ra:
-
interleukin-1 receptor antagonist protein
- MMP:
-
matrix metalloproteinase
- OA:
-
osteoarthritis
- TIMP:
-
tissue inhibitor of metalloproteinase
References
Alwan WH, Carter SD, Bennett D, Edwards GB . Glycosaminoglycans in horses with osteoarthritis . Equine Vet J 1991 ; 23 : 44 - 47
Backhaus R, Zehe C, Wegehingel S, Kehlenbach A, Schwappach B, Nickel W . Unconventional protein secretion: membrane translocation of FGF-2 does not require protein unfolding . J Cell Sci 2004 ; 117 : 1727 - 1736
Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle JP, Martelly I, Papy-García D . Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies . Glycobiology 2003 ; 13 : 647 - 653
Berenbaum F . New horizons and perspectives in the treatment of osteoarthritis . Arthritis Res Ther 2008 ; 10 : S1 -
Blom AB, van der Kraan PM, van den Berg WB . Cytokine targeting in osteoarthritis . Curr Drug Targets 2007 ; 8 : 283 - 292
Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T . Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis . Arthritis Rheum 2009 ; 60 : 2019 - 2027
Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, Rueger DC, Loeser RF . Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads . Osteoarthritis Cartilage 2007 ; 15 : 421 - 430
Cuevas P, Burgos J, Baird A . Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo . Biochem Biophys Res Commun 1988 ; 156 : 611 - 618
De Ceuninck F . The birth and infancy of proteomic analysis in osteoarthritis research . Curr Opin Mol Ther 2007 ; 9 : 263 - 269
Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC . Osteoarthritis gene therapy . Gene Ther 2004 ; 11 : 379 - 389
Evans CH . Gene therapy: What have we accomplished and where do we go from here ? J Rheumatol Suppl 2005 ; 72 : 17 - 20
Evans CH, Gouze E, Gouze JN, Robbins PD, Ghivizzani SC . Gene therapeutic approaches-transfer in vivo . Adv Drug Deliv Rev 2006 ; 58 : 243 - 258
Fosang AJ, Rogerson FM . Identifying the human aggrecanase . Osteoarthritis Cartilage 2010 . [Epub ahead of print]
Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW . Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene . Gene Ther 2002 ; 9 : 12 - 20
Fujimoto E, Ochi M, Kato Y, Mochizuki Y, Sumen Y, Ikuta Y . Beneficial effect of basic fibroblast growth factor on the repair of full-thickness defects in rabbit articular cartilage . Arch Orthop Trauma Surg 1999 ; 119 : 139 - 145
Ge Z, Hu Y, Heng BC, Yang Z, Ouyang H, Lee EH, Cao T . Osteoarthritis and therapy . Arthritis Rheum 2006 ; 55 : 493 - 500
Ghivizzani SC, Oligino TJ, Glorioso JC, Robbins PD, Evans CH . Direct gene delivery strategies for the treatment of rheumatoid arthritis . Drug Discov Today 2001 ; 6 : 259 - 267
Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML . Construction of adenovirus vectors through Cre-lox recombination . J Virol 1997 ; 71 : 1842 - 1849
Haupt JL, Frisbie DD, McIlwraith CW, Robbins PD, Ghivizzani S, Evans CH, Nixon AJ . Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model . J Orthop Res 2005 ; 23 : 118 - 126
Hendriks J, Riesle J, Vanblitterswijk CA . Effect of stratified culture compared to confluent culture in monolayer on proliferation and differentiation of human articular chondrocytes . Tissue Eng 2006 ; 12 : 2397 - 2405
Hillgenberg M, Hofmann C, Stadler H, Löser P . High-efficiency system for the construction of adenovirus vectors and its application to the generation of representative adenovirus-based cDNA expression libraries . J Virol 2006 ; 80 : 5435 - 5450
Hiraide A, Yokoo N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Saito T . Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector . Hum Gene Ther 2005 ; 16 : 1413 - 1421
Hsieh JL, Shen PC, Shiau AL, Jou IM, Lee CH, Wang CR, Teo ML, Wu CL . Intraarticular gene transfer of thrombospondin-1 suppresses the disease progression of experimental osteoarthritis . J Orthop Res 2010 . [Epub ahead of print]
Inoue A, Takahashi KA, Arai Y, Tonomura H, Sakao K, Saito M, Fujioka M, Fujiwara H, Tabata Y, Kubo T . The therapeutic effects of basic fibroblast growth factor contained in gelatin hydrogel microspheres on experimental osteoarthritis in the rabbit knee . Arthritis Rheum 2006 ; 54 : 264 - 270
Kato Y, Hiraki Y, Inoue H, Kinoshita M, Yutani Y, Suzuki F . Differential and synergistic actions of somatomedin-like growth factors, fibroblast growth factor and epidermal growth factor in rabbit costal chondrocytes . Eur J Biochem 1983 ; 129 : 685 - 690
Kato Y, Gospodarowicz D . Sulfated proteoglycan synthesis by confluent cultures of rabbit costal chondrocytes grown in the presence of fibroblast growth factor . J Cell Biol 1985 ; 100 : 477 - 485
Kawabata K, Sakurai F, Koizumi N, Hayakawa T, Mizuguchi H . Adenovirus vector-mediated gene transfer into stem cells . Mol Pharm 2006 ; 3 : 95 - 103
Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB . Current concepts in the pathogenesis of osteoarthritis . Osteoarthritis Cartilage 2008 ; 16 : S1 - S3
Lin HT, Tsai HY, Liu CP, Yuan TT . Comparability of bovine virus titers obtained by TCID50/ml and FAID50/ml . J Virol Methods 2010 ; 165 : 121 - 124
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method . Methods 2001 ; 25 : 402 - 408
Lohmander LS, Dalén N, Englund G, Hämäläinen M, Jensen EM, Karlsson K, Odensten M, Ryd L, Sernbo I, Suomalainen O, Tegnander A . Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: A randomised, double blind, placebo controlled multicentre trial . Ann Rheum Dis 1996 ; 55 : 424 - 431
Malemud CJ . Cytokines as therapeutic targets for osteoarthritis . Biodrugs 2004 ; 18 : 23 - 35
Maniwa S, Ochi M, Motomura T, Nishikori T, Chen J, Naora H . Effects of hyaluronic acid and basic fibroblast growth factor on motility of chondrocytes and synovial cells in culture . Acta Orthop Scand 2001 ; 72 : 299 - 303
Mankin HJ, Dorfman H, Lippiello L, Zarins A . Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data . J Bone Joint Surg Am 1971 ; 53 : 523 - 537
Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ . Cartilage in normal and osteoarthritis conditions . Best Pract Res Clin Rheumatol 2008 ; 22 : 351 - 384
Mi Z, Ghivizzani SC, Lechman ER, Jaffurs D, Glorioso JC, Evans CH, Robbins PD . Adenovirus-mediated gene transfer of insulin-like growth factor 1 stimulates proteoglycan synthesis in rabbit joints . Arthritis Rheum 2000 ; 43 : 2563 - 2570
Miyakoshi N, Kobayashi M, Nozaka K, Okada K, Shimada Y, Itoi E . Effects of intraarticular administration of basic fibroblast growth factor with hyaluronic acid on osteochondral defects of the knee in rabbits . Arch Orthop Trauma Surg 2005 ; 125 : 683 - 692
Nakajima F, Nakajima A, Ogasawara A, Moriya H, Yamazaki M . Effects of a single percutaneous injection of basic fibroblast growth factor on the healing of a closed femoral shaft fracture in the rat . Calcif Tissue Int 2007 ; 81 : 132 - 138
Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, Fernandes JC, Martel-Pelletier J . In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy . Arthritis Rheum 1997 ; 40 : 1012 - 1019
Piecha D, Weik J, Kheil H, Becher G, Timmermann A, Jaworski A, Burger M, Hofmann MW . Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants . Inflamm Res 2010 ; 59 : 379 - 389
Poole AR, Ionescu M, Swan A, Dieppe PA . Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan. Implications for pathogenesis . J Clin Invest 1994 ; 94 : 25 - 33
Punzi L, Oliviero F, Plebani M . New biochemical insights into the pathogenesis of osteoarthritis and the role of laboratory investigations in clinical assessment . Crit Rev Clin Lab Sci 2005 ; 42 : 279 - 309
Sah RL, Chen AC, Grodzinsky AJ, Trippel SB . Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants . Arch Biochem Biophys 1994 ; 308 : 137 - 147
Sawaji Y, Hynes J, Vincent T, Saklatvala J . Fibroblast growth factor 2 inhibits induction of aggrecanase activity in human articular cartilage . Arthritis Rheum 2008 ; 58 : 3498 - 3509
Shirakawa T . Clinical trial design for adenoviral gene therapy products . Drug News Perspect 2009 ; 22 : 140 - 145
Sorensen V, Nilsen T, Wiedlocha A . Functional diversity of FGF-2 isoforms by intracellular sorting . Bioessays 2006 ; 28 : 504 - 514
Taverna S, Ghersi G, Ginestra A, Rigogliuso S, Pecorella S, Alaimo G, Saladino F, Dolo V, Dell'Era P, Pavan A, Pizzolanti G, Mignatti P, Presta M, Vittorelli ML . Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells . J Biol Chem 2003 ; 278 : 51911 - 51919
van der Kraan PM, van den Berg WB . Osteophytes: relevance and biology . Osteoarthritis Cartilage 2007 ; 15 : 237 - 244
Weisser J, Rahfoth B, Timmermann A, Aigner T, Bräuer R, von der Mark K . Role of growth factors in rabbit articular cartilage repair by chondrocytes in agarose . Osteoarthritis Cartilage 2001 ; 9 : S48 - S54
Wu CW, Morrell MR, Heinze E, Concoff AL, Wollaston SJ, Arnold EL, Singh R, Charles C, Skovrun ML, FitzGerald JD, Moreland LW, Kalunian KC . Validation of American College of Rheumatology classification criteria for knee osteoarthritis using arthroscopically defined cartilage damage scores . Semin Arthritis Rheum 2005 ; 35 : 197 - 201
Yamamoto T, Wakitani S, Imoto K, Hattori T, Nakaya H, Saito M, Yonenobu K . Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits . Osteoarthritis Cartilage 2004 ; 12 : 636 - 641
Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, Mizukami H, Ozawa K, Koshino T . Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector . Arthritis Rheum 2005 ; 52 : 164 - 170
Yoshioka M, Coutts RD, Amiel D, Hacker SA . Characterization of a model of osteoarthritis in the rabbit knee . Osteoarthritis Cartilage 1996 ; 4 : 87 - 98
Zehe C, Engling A, Wegehingel S, Schäfer T, Nickel W . Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2 . Proc Natl Acad Sci U S A 2006 ; 103 : 15479 - 15484
Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD, Tang D, Sheu TJ, Li TF, Shi Q, Wang YJ . IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways . Osteoarthritis Cartilage 2009 ; 17 : 100 - 106
Zhang XL, Mao ZB, Yu CL . Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10 . J Orthop Res 2004 ; 22 : 742 - 750
Acknowledgements
This research was supported by the Natural Science Foundation of China (30672141 and 30973539), the International Scientific and Technological Cooperation Project of China (30710329) and the Hubert Curien Project of France and China Cooperation (20999RC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chen, B., Qin, J., Wang, H. et al. Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med 42, 684–695 (2010). https://doi.org/10.3858/emm.2010.42.10.067
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2010.42.10.067
Keywords
This article is cited by
-
Enhancement of tendon-to-bone healing after anterior cruciate ligament reconstruction using bone marrow-derived mesenchymal stem cells genetically modified with bFGF/BMP2
Scientific Reports (2016)
-
Origin and function of cartilage stem/progenitor cells in osteoarthritis
Nature Reviews Rheumatology (2015)
-
Tgfbi Deficiency Leads to a Reduction in Skeletal Size and Degradation of the Bone Matrix
Calcified Tissue International (2015)
-
rAAV-mediated overexpression of TGF-β stably restructures human osteoarthritic articular cartilage in situ
Journal of Translational Medicine (2013)
-
Stem cells and other innovative intra-articular therapies for osteoarthritis: what does the future hold?
BMC Medicine (2012)