Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Original Article
Clinicopathological Analysis of 21 Thymic Neuroendocrine Tumors - Soomin Ahn, Jae Jun Lee, Sang Yun Ha, Chang Ohk Sung,, Jhingook Kim1, Joungho Han
-
Korean Journal of Pathology 2012;46(3):221-225.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.221
Published online: June 22, 2012
Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
1Department of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding Author: Joungho Han, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2765, Fax: +82-2-3410-0025, 'Joungho.han@samsung.net'
- *Present address of Chang Ohk Sung: Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Thymic neuroendocrine carcinomas (NECs) are uncommon, for which there is no established information available because of a limited number of epidemiological study in Asia.
-
Methods
- We reviewed 21 cases of surgically resected thymic NECs, and evaluated their pathological and clinical features.
-
Results
- It showed male predominance (male/female ratio, 15/6) with wide age range from 20 to 72 years (mean age, 49 years). All 21 cases were divided into two types according to the World Health Organization criteria: atypical carcinoid (n=18) and large cell NEC (n=3). Three cases of atypical carcinoid (AC) were associated with ectopic Cushing's syndrome. All the patients (3/3) with large cell NEC (3/3) and 16.7% (3/18) of those with AC died of tumor progression. Common sites of metastasis included lung, lymph node, brain, lumbar spine, mediastinum, bone, and liver.
-
Conclusions
- In conclusion, thymic neuroendocrine tumors carry a poor prognosis. Regarding the tumor classification, our results showed that a vast majority of carcinoids in the thymus correspond to ACs. In addition, our results also indicate that typical carcinoid is a very rare entity. Some cases of AC exhibited a large size, solid pattern and they showed aggressive clinical behavior, which highlights the spectrum of histologic appearances of thymic NECs.
- Cases
- During a 16-year period (1995-2010), a total of 21 patients with primary thymic neuroendocrine tumors had been surgically treated at the department of thoracic and cardiovascular surgery of Samsung Medical Center in Seoul, Korea. Note that these 21 cases included no cases of pulmonary neuroendocrine tumors and tumors involving both lung and thymus. Clinical data and follow-up data were obtained through a retrospective analysis of the medical records, and these data include sex, age, initial symptoms and signs, a notable past history, gross findings, treatment and follow-ups. Adequate information was obtained for all the 21 cases. The postoperative staging was done based on the classification system of Masaoka et al.10 for thymomas. All the patients were followed up until January of 2012 during a median follow-up period of 34.95 months.
- Histological classification
- For histological classification, the histopathologic features were assessed and these include tumor differentiation, mitotic rate, presence or absence of necrosis, presence of local invasion and lymph node metastasis. The tumors were classified into four types: TC, AC, LCNEC, and SCNEC according to the WHO criteria based on the histopathologic differentiation as shown below:
-
TC: A well-differentiated tumor with mitotic figures, fewer than 2 mitoses per 10 high power fields (HPFs) and no necrosis
AC: A well-differentiated tumor with mitotic figures, 2-10 mitoses per 10 HPFs and/or presence of necrosis
SCNEC: A poorly-differentiated tumor with small cell cytology, mitotic figures with more than 10 mitoses per 10 HPFs and extensive areas of necrosis
LCNEC: A poorly-differentiated tumor with non-small cell NEC, mitotic figures with more than 10 mitoses per 10 HPFs and extensive areas of necrosis.6
- Immunohistochemistry
- In the current study, we used representative formalin-fixed, paraffin-embedded tissue sections for the immunohistochemical staining in our series of 21 cases of primary thymic neuroendocrine tumors. To evaluate neuroendocrine differentiation of the thymic neoplasm, synaptophysin, chromogranin or CD56 were chosen as neuroendocrine markers. Immunohistochemical staining was performed on 3-µm thick sections from each case using a biotin-avidin-peroxidase method on a BOND-MAX autostainer (Leica, Wetzlar, Germany) after retrieval with T/E buffer (CD56) or citrate buffer (chromogranin and synaptophysin). We also used primary antibodies to synaptophysin (1:100, Dako, Glostrup, Denmark), chromogranin (1:400, Dako), CD56 (1: 200, Novocastra, Newcastle upon Tyne, UK) and adrenocorticotrophic hormone (ACTH; 1:100, Dako).
MATERIALS AND METHODS
- Clinical features
- In our series, there was a male predilection with a male-to-female ratio of 15:6. In addition, the mean age of patients was 49 years (range, 20 to 72 years). Three cases of AC were associated with ectopic Cushing's syndrome. Clinical and radiologic impressions were thymoma or lymphoma preoperatively in most cases of our series. After the resection, adjuvant therapy was performed according to our treatment protocol. Postoperatively, the concurrent chemoradiation therapy (CCRT) and the radiotherapy (RT) alone were preferentially performed for cases of LCNEC and AC, respectively. During the follow up period, a local recurrence or a distant metastasis were observed in 100% (3/3) of patients with LCNEC and 27.8% (5/18) of those with AC. Common sites of metastasis included lung, lymph node, brain, lumbar spine, mediastinum, bone, and liver. The cause of death was tumor progression in 100% (3/3) of patients with LCNEC and 16.7% (3/18) of those with AC. Clinical features are summarized in Table 1.
- Histological features
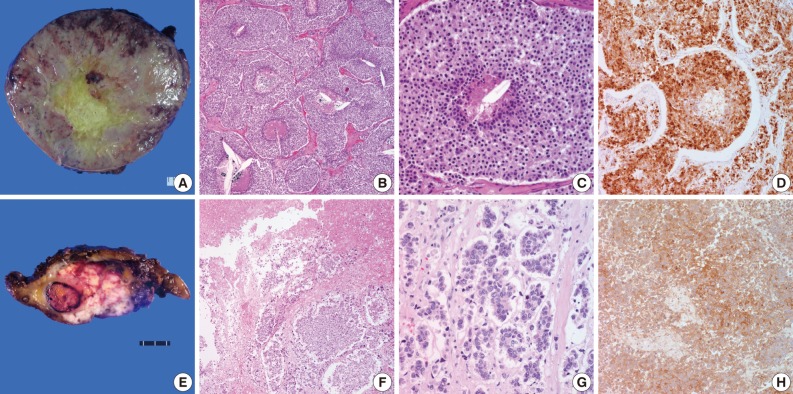
- All 21 cases were divided into two types according to the WHO criteria: AC (n=18) and large cell NEC (n=3). In addition, there were no cases of TC tumor. Histological features are summarized in Table 2. The tumors were described as a large, soft and whitish-tan to brown mass with the greatest diameter of 1.8-21 cm (Fig. 1A). The mean size of the mass was 6.81 cm and 8.27 cm in cases of AC and LCNEC, respectively. Some of the tumors showed an infiltrative growth pattern to the adjacent organs such as the pericardium. On cut sections, the tumors revealed focal areas of hemorrhage and necrosis. In particular, LCNEC showed extensive areas of necrosis, which is the hallmark of poorly-differentiated neuroendocrine thymic carcinomas (Fig. 1E). AC showed a punctate necrosis with a maximum of 13 (0-10) mitoses or it was devoid of necrosis but showed a proliferation with a mitotic rate of up to 9 mitoses per 10 HPF (Fig. 1B, C). Thymic LCNEC was defined as a poorly-differentiated non-small cell NEC with a mitotic rate of >10 mitoses per 10 HPF. The mean number of mitotic cells per 10 HPF was 32. It was notable that there was an extensive necrosis (Fig. 1F, G). Of all the 18 cases of AC, 11 frequently revealed lymphatic emboli and six, undergoing a lymph node dissection, demonstrated a nodal metastasis. All the cases of LCNEC frequently showed lymphatic emboli. Of these, one case proved to histologically have a nodal metastasis. Finally, tumors associated with ectopic hormone secretion were also found to share the characteristic features of cytoplasm. That is, tumor cells had focally abundant clear cytoplasm.
- Immunohistochemical findings
- Neuroendocrine differentiation was revealed by positive immunohistochemistry for neuroendocrine markers including synaptophysin, chromogranin and CD56. In all the cases, at least two of these markers were expressed. They showed diffuse membranous and cytoplasmic, dot-like staining pattern. There was no definite difference in the degree of immunohistochemical staining between cases of LCNEC and those of AC (Fig. 1D). But the degree of immunohistochemical staining was lower in cases of LCNEC as compared with those of AC (Fig. 1H). In addition, we also performed an immunohistochemical staining of ACTH for tumors with ectopic ACTH secretion. All the three cases showed a nuclear positivity.
RESULTS
- We performed a clinicopathologic examination of primary thymic neuroendocrine tumors. Thymic epithelial tumors, predominantly or exclusively composed of neuroendocrine cells, are classified as NECs of the thymus. Thymic NECs are rare, constituting 2-5% of thymic epithelial tumors.6 There is a sufficient amount of statistical data for pulmonary NECs. But there is a paucity of data about the clinicopathologic correlations among the thymic NECs. Considering this, the first edition of the WHO classification of tumors of the thymus suggests that pathologists classify thymic neuroendocrine tumors based on the same criteria as NECs of the lung.6 It is known that a vast majority of carcinoids in the thymus would correspond to ACs if the same criteria are applied as the lung.6 In addition, several studies have reported that a substantial number of cases of AC are associated with ectopic hormone secretion.5,11,12 In the current study, our clinical series of patients (n=21) comprised 18 cases of AC and 3 cases of LCNEC. In addition, three cases of AC had endocrine manifestations. Furthermore, these three cases of AC were accompanied by Cushing's syndrome. It is well known that AC shows a better clinical behavior than LCNEC. However there were two patients with AC, associated with ectopic hormone secretion, both of whom died of tumor progression and metastasis although they had a small-sized tumor and low mitotic counts. Furthermore, there was another patient with ectopic hormone secretion who developed a pulmonary metastasis. In conclusion, our results indicate that ACs associated with ectopic hormone secretion show a poorer prognosis as compared with conventional ones. In our series, there were no cases of TC. This implies that TC is a very rare entity unlike the pulmonary NECs. Over the past few decades, a three-tiered pathologic classification has been used and NECS are classified into low grade, intermediate-grade, and high-grade ones.13,14 Based on this classification system, some previously diagnosed cases of low- or intermediate-grade NECs actually correspond to AC. Those who were diagnosed with low or intermediate grade neuroendocrine carcinoma underwent post-operative CCRT while only post-operative RT was added to AC patients according to the current treatment trend.
- With regard to the clinical behavior of NECs, our results are in agreement with the previous reports that thymic neuroendocrine tumors show a poor prognosis. During the follow-up period, 100% (3/3) of patients with LCNEC and 16.7% (3/18) of those with AC died of tumor progression. However no statistical analysis has been attempted to determine whether the classification of tumors is not dependent on a prognosis of patients because of a limited number of cases enrolled in the current study.
- Thymic neuroendocrine tumors are histologically classified according to tumor differentiation, presence or absence of necrosis and mitotic counts.6 ACs are therefore classified as a carcinoid tumor having architectural features of the classic type but exhibiting 2-10 mitoses per 10 HPF and/or foci of necrosis. AC is actually classified as a group of well-differentiated NEC. In our series, Cases 12, 15, and 16 were classified as an AC despite a high mitotic index of >10 mitoses per 10 HPF. In these cases, high mitotic counts may pose a diagnostic challenge to pathologists. However, tumor differentiation is one of the most important factors by which pathologists can make a differential diagnosis of carcinoid tumor from SCNEC and LCNEC. According to the WHO criteria, both TC and AC are classified a well-differentiated tumor and both SCNEC and LCNEC are classified a poorly differentiated tumor. Cases 12, 15, and 16 showed the similar characteristics of carcinoid tumor and they had a lack of the typical histologic characteristics of LCNEC such as extensive necrosis or poorly-differentiated pattern. Not including high mitotic counts, the histologic findings did not seem to correlate with a poorly-differentiated carcinoma which usually reveals aggressive clinical behavior. In addition, some tumors exhibited large tumor size and mitotic counts varied depending on the part of the tumor. We thought that high mitotic counts could be seen as the tumor grew. Therefore we classified such tumors as an AC rather than a LCNEC. Considering all LCNEC patients died of the tumor, the fact those patients were alive without evidence of relapse might support out diagnosis. Our results indicate that mitotic counts do not fully reflect morphology of the tumor. It can also be presumed that the current WHO classification needs a more detailed explanation because it might pose a diagnostic challenge to pathologists. Further studies are therefore warranted to examine the tumor classification.
- The clinical behavior of AC varies in several studies. It has been reported that even "innocent" looking and encapsulated carcinoids bear a significant risk for recurrence, metastasis and tumor-associated death.6 On the other hand, one of the recent studies has shown a better prognosis of atypical thymic carcinoids as compared to pulmonary carcinoids.6 In our series, 16.7% (3/18) of patients with AC patients died of tumor progression, two of whom had a concurrent presence of ectopic hormone secretion. This implies that hormone-expressing tumors show an unfavorable prognosis.
- Further, regarding the view that thymic neuroendcrine tumors are clinically more aggressive than morphologically identical neuroendocrine tumors of the lung, we also observed that primary thymic LCNECs tended to exhibit less neuroendocrine differentiation, such as trabeculae, nesting, rossettes and perilobular palisading patterns, which is commonly seen in pulmonary LCNECs. This may suggest that thymic LCNECs are higher-grade tumors than pulmonary LCNECs.
- In conclusion, thymic neuroendocrine tumors carry a poor prognosis. Regarding the tumor classification, our results showed that a vast majority of carcinoids in the thymus correspond to ACs. In addition, our results also indicate that TC is a very rare entity. Some cases of AC exhibited a large size, solid pattern and they showed aggressive clinical behavior, which highlights the spectrum of histologic appearances of thymic NECs.
DISCUSSION
- 1. Yamauchi S, Yamada Y, Tsushima T, et al. Thymic carcinoid tumor with Cushing syndrome. Kyobu Geka 2008; 61: 143–146. PMID: 18268953. PubMed
- 2. Mega S, Oguri M, Kawasaki R, Hazama K, Iwai K, Kondo S. Large-cell neuroendocrine carcinoma in the thymus. Gen Thorac Cardiovasc Surg 2008; 56: 566–569. PMID: 19002759. ArticlePubMed
- 3. Haga T, Nakajima Y, Kitamura A, Kuroda F, Takiguchi Y, Tatsumi K. A rare case of large cell neuroendocrine carcinoma of the thymus. Nihon Kokyuki Gakkai Zasshi 2010; 48: 755–758. PMID: 21066864. PubMed
- 4. Rena O, Filosso PL, Maggi G, Casadio C. Neuroendocrine tumors (carcinoid) of the thymic gland. Ann Thorac Surg 2003; 75: 633PMID: 12607697. Article
- 5. Van Brandt V, Heyman S, Van Marck E, Hendriks J, Van Schil P. Atypical presentation of an atypical carcinoid. Ann Thorac Surg 2009; 88: 2004–2006. PMID: 19932277. ArticlePubMed
- 6. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus and heart. 2004; Lyon: IARC Press.
- 7. Tiffet O, Nicholson AG, Ladas G, Sheppard MN, Goldstraw P. A clinicopathologic study of 12 neuroendocrine tumors arising in the thymus. Chest 2003; 124: 141–146. PMID: 12853516. ArticlePubMed
- 8. Cardillo G, Treggiari S, Paul MA, et al. Primary neuroendocrine tumours of the thymus: a clinicopathologic and prognostic study in 19 patients. Eur J Cardiothorac Surg 2010; 37: 814–818. PMID: 19954997. ArticlePubMed
- 9. Moran CA. Primary neuroendocrine carcinomas of the mediastinum: review of current criteria for histopathologic diagnosis and classification. Semin Diagn Pathol 2005; 22: 223–229. PMID: 16711403. ArticlePubMed
- 10. Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981; 48: 2485–2492. PMID: 7296496. ArticlePubMed
- 11. Wang WQ, Ye L, Bi YF, et al. Six cases of ectopic ACTH syndrome caused by thymic carcinoid. J Endocrinol Invest 2006; 29: 293–297. PMID: 16699293. ArticlePubMed
- 12. Simsek I, Pay S, Dinc A, Erdem H, Kurt B. Atypical carcinoid tumor of the thymus with ectopic ACTH production developed during the course of etanercept treatment: case report. Clin Rheumatol 2007; 26: 1561–1562. PMID: 17061154. ArticlePubMed
- 13. Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus: a clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000; 114: 100–110. PMID: 10884805. ArticlePubMed
- 14. Rosai J, Levine G, Weber WR, Higa E. Carcinoid tumors and oat cell carcinomas of the thymus. Pathol Annu 1976; 11: 201–226. PMID: 188002. PubMed
References


RFS, relapse-free survival; OS, overall survival; F, female; Local sx, local symptom; AC, atypical carcinoid; R0, complete resection; preopRT, preoperative radiotherapy; postopCT, postoperative chemotherapy; DM, distant metastasis; DOD, died of disease; M, male; ASx, asymptomatic; postopRT, postoperative radiotherapy; N, none; ADF, alive disease-free; postopCCRT, postoperative concurrent chemoradiation therapy; R1, microscopic residual disease; preopCT, preoperative chemotherapy; AWD, alive with disease; ACTH, adrenocorticotropic hormone; LR, local recurrence; LCNEC, large cell neuroendocrine carcinoma; preopCCRT, preoperative concurrent chemoradiation therapy.
Figure & Data
References
Citations

- Thymic neuroendocrine tumours
Jan von der Thüsen
Diagnostic Histopathology.2023; 29(2): 114. CrossRef - The Utility of Fine Needle Aspiration (FNA) Biopsy in the Diagnosis of Mediastinal Lesions
Uma Kundu, Qiong Gan, Deepak Donthi, Nour Sneige
Diagnostics.2023; 13(14): 2400. CrossRef - Paediatric and adolescent ectopic Cushing's syndrome: systematic review
Chethan Yami Channaiah, Manjiri Karlekar, Vijaya Sarathi, Anurag Ranjan Lila, Shruthi Ravindra, Padma Vikram Badhe, Gaurav Malhotra, Saba Samad Memon, Virendra Ashokrao Patil, C S Pramesh, Tushar Bandgar
European Journal of Endocrinology.2023; 189(4): S75. CrossRef - Multiple endocrine neoplasia type 1 (MEN-1) and neuroendocrine neoplasms (NENs)
Grigoris Effraimidis, Ulrich Knigge, Maria Rossing, Peter Oturai, Åse Krogh Rasmussen, Ulla Feldt-Rasmussen
Seminars in Cancer Biology.2022; 79: 141. CrossRef - Multiple electrolyte disturbances as the presenting feature of multiple endocrine neoplasia type 1 (MEN-1)
Adrian Po Zhu Li, Sheela Sathyanarayan, Salvador Diaz-Cano, Sobia Arshad, Eftychia E Drakou, Royce P Vincent, Ashley B Grossman, Simon J B Aylwin, Georgios K Dimitriadis
Endocrinology, Diabetes & Metabolism Case Reports.2022;[Epub] CrossRef - Metastatic Thymic Carcinoid: Does Surgeon Have a Primary Role?
Kumud Gupta, Ravindra K. Dewan, Vinitha Viswambharan Nair, Rajat Saxena, Shaleen Prasad
The Indian Journal of Chest Diseases and Allied Sciences.2022; 56(4): 255. CrossRef - A resected case of large cell neuroendocrine carcinoma of the thymus
Masashi Umeda, Takahiko Misao, Tomoya Senoh, Yoshinobu Shikatani, Motoi Aoe
The Journal of the Japanese Association for Chest Surgery.2022; 36(7): 766. CrossRef - Treatment strategy and prognostic analysis of spinal metastases from thymomas: A retrospective study from a single center
Shuzhong Liu, Xi Zhou, An Song, Zhen Huo, Siyuan Yao, Yipeng Wang, Yong Liu
Clinical Neurology and Neurosurgery.2020; 196: 106056. CrossRef - Large Cell Neuroendocrine Carcinoma of the Mediastinum Successfully Treated with Systemic Chemotherapy after Palliative Radiotherapy
Takeaki Hidaka, Saki Okuzumi, Ako Matsuhashi, Hidenori Takahashi, Kazunori Hata, Seiichiro Shimizu, Yoshinobu Iwasaki
Internal Medicine.2019; 58(4): 563. CrossRef - Surgical management of spinal metastases of thymic carcinoma
Shuzhong Liu, Xi Zhou, An Song, Zhen Huo, William A. Li, Radhika Rastogi, Yipeng Wang, Yong Liu
Medicine.2019; 98(3): e14198. CrossRef - Resected thymic large cell neuroendocrine carcinoma: A case report and review of the literature
Shogo Ogata, Ryo Maeda, Masaki Tomita, Yuichiro Sato, Takanori Ayabe, Kunihide Nakamura
International Journal of Surgery Case Reports.2019; 60: 53. CrossRef - Metastatic or locally advanced mediastinal neuroendocrine tumours
Aadil Adnan, Shreyas Kudachi, Sudha Ramesh, Kumar Prabhash, Sandip Basu
Nuclear Medicine Communications.2019; 40(9): 947. CrossRef - Results of treatment for thymic neuroendocrine tumours: multicentre clinicopathological study†
Naoko Ose, Hajime Maeda, Masayoshi Inoue, Eiichi Morii, Yasushi Shintani, Hiroshi Matsui, Hirohito Tada, Tositeru Tokunaga, Kenji Kimura, Yasushi Sakamaki, Yukiyasu Takeuchi, Kenjiro Fukuhara, Hiroshi Katsura, Teruo Iwasaki, Meinoshin Okumura
Interactive CardioVascular and Thoracic Surgery.2018; 26(1): 18. CrossRef - Patterns of Failure Following Postoperative Radiation Therapy Based on “Tumor Bed With Margin” for Stage II to IV Type C Thymic Epithelial Tumor
Kyung Hwa Lee, Jae Myoung Noh, Yong Chan Ahn, Dongryul Oh, Jhingook Kim, Young Mog Shim, Jung-ho Han
International Journal of Radiation Oncology*Biology*Physics.2018; 102(5): 1505. CrossRef - Resected thymic large cell neuroendocrine carcinoma: report of a case
Hiromitsu Domen, Yasuhiro Hida, Masaaki Sato, Haruka Takahashi, Tatsuru Ishikawa, Yosuke Shionoya, Midori Hashimoto, Kaoru Nishiyama, Yuma Aoki, Kazuho Inoko, Syotaro Furukawa, Kazuomi Ichinokawa, Hidehisa Yamada
Surgical Case Reports.2018;[Epub] CrossRef - Successful treatment of malignant thymoma with sacrum metastases
Shuzhong Liu, Xi Zhou, An Song, Zhen Huo, William A. Li, Radhika Rastogi, Yipeng Wang, Yong Liu
Medicine.2018; 97(51): e13796. CrossRef - Incidental metastatic mediastinal atypical carcinoid in a patient with parathyroid adenoma: a case report
Zareen Kiran, Asma Ahmed, Owais Rashid, Saira Fatima, Faizan Malik, Saulat Fatimi, Mubassher Ikram
Journal of Medical Case Reports.2017;[Epub] CrossRef - Thymus neuroendocrine tumors with CTNNB1 gene mutations, disarrayed ß-catenin expression, and dual intra-tumor Ki-67 labeling index compartmentalization challenge the concept of secondary high-grade neuroendocrine tumor: a paradigm shift
Alessandra Fabbri, Mara Cossa, Angelica Sonzogni, Paolo Bidoli, Stefania Canova, Diego Cortinovis, Maria Ida Abbate, Fiorella Calabrese, Nazarena Nannini, Francesca Lunardi, Giulio Rossi, Stefano La Rosa, Carlo Capella, Elena Tamborini, Federica Perrone,
Virchows Archiv.2017; 471(1): 31. CrossRef - Thymic large cell neuroendocrine carcinoma – a rare and aggressive tumor: a case report
Efared Boubacar, Gabrielle Atsame-Ebang, Sani Rabiou, Ammor Fatimazahra, Asmae Mazti, Ibrahim S. Sidibé, Layla Tahiri, Nawal Hammas, Ouadnouni Yassine, Smahi Mohamed, Chbani Laila, El Fatemi Hinde
Journal of Medical Case Reports.2017;[Epub] CrossRef - Clinicopathological features of neoplasms with neuroendocrine differentiation occurring in the liver
Yoriko Nomura, Osamu Nakashima, Jun Akiba, Sachiko Ogasawara, Shogo Fukutomi, Rin Yamaguchi, Hironori Kusano, Masayoshi Kage, Koji Okuda, Hirohisa Yano
Journal of Clinical Pathology.2017; 70(7): 563. CrossRef - Retrosternal goiter and thymic carcinoid: A rare co-existence
Abdulsalam Yaseen Taha, Nezar A. Almahfooz, Hassanain H. Khudair
Journal of the Egyptian Society of Cardio-Thoracic Surgery.2017; 25(4): 369. CrossRef - A case of large-cell neuroendocrine carcinoma of the thymus involving a patient with long-term survival after surgery
Qiuming Kan, Kohei Tagawa, Teruaki Ishida, Mitsuyo Nishimura, Katsuhiko Aoyama
The Journal of the Japanese Association for Chest Surgery.2017; 31(7): 927. CrossRef - Neuroendokrine Neoplasien des Mediastinums
L. Brcic, M. Heidinger, H. Popper
Der Pathologe.2016; 37(5): 434. CrossRef - Outcome of primary neuroendocrine tumors of the thymus: A joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases
Pier Luigi Filosso, Xiaopan Yao, Usman Ahmad, Yilei Zhan, James Huang, Enrico Ruffini, William Travis, Marco Lucchi, Andreas Rimner, Alberto Antonicelli, Francesco Guerrera, Frank Detterbeck
The Journal of Thoracic and Cardiovascular Surgery.2015; 149(1): 103. CrossRef - Clinical Significance of Persistent Tumor in Bone Marrow during Treatment of High-risk Neuroblastoma
Young Bae Choi, Go Eun Bae, Na Hee Lee, Jung-Sun Kim, Soo Hyun Lee, Keon Hee Yoo, Ki Woong Sung, Hong Hoe Koo
Journal of Korean Medical Science.2015; 30(8): 1062. CrossRef - Tumor genetics and survival of thymic neuroendocrine neoplasms: A multi‐institutional clinicopathologic study
Philipp Ströbel, Andreas Zettl, Konstantin Shilo, Wen‐Yu Chuang, Andrew G. Nicholson, Yoshihiro Matsuno, Anthony Gal, Rolf Hubert Laeng, Peter Engel, Carlo Capella, Mirella Marino, John Kwok-Cheung Chan, Andreas Rosenwald, William Travis, Teri J. Franks,
Genes, Chromosomes and Cancer.2014; 53(9): 738. CrossRef - Disseminated large cell neuroendocrine carcinoma associated with ectopic adrenocorticotropic hormone secretion
A Van der Walt, K Huddle, S Pather, A Korb
Journal of Endocrinology, Metabolism and Diabetes of South Africa.2014; 19(1): 40. CrossRef - Morphologic Alteration of Metastatic Neuroblastic Tumor in Bone Marrow after Chemotherapy
Go Eun Bae, Yeon-Lim Suh, Ki Woong Sung, Jung-Sun Kim
Korean Journal of Pathology.2013; 47(5): 433. CrossRef

 E-submission
E-submission

 PubReader
PubReader Cite this Article
Cite this Article